Opsin evolution: informative indels
See also: Curated Sequences | Ancestral Introns | Cytoplasmic face | Ancestral Sequences | Alignment | Update Blog
Introduction to indels
Insertions and deletions of amino acids (together called coding indels) are a class of genetic event rarely fixed in conserved protein sequence regions. It is not immediately clear whether a given indel represents an insertion or a deletion. The process of deciding is called indel resolution; it requires a phylogenetic tree allowing determination of ancestral length. If outgroups are consistently short, then by parsimony the ingroup clade with longer length experienced an insertion. Indels are unresolvable when outgroup data is not available. Two or more consistent outgroup nodes establishes a period of length stability.
It is implausible -- rarity cubed -- that multiple outgroups plus an ingroup experienced independent deletions of the same length at the same site (though the exact site can be difficult to evaluate if flanking residues were also affected by the original genetic event or subsequently by accelerated compensatory mutation). Advanced statistical methods can provide only illusory gains over simple parsimony because the underlying required models of indel formation are entirely speculative.
Nonetheless, examples of homoplasy are easy to come by, especially in repetitive nucleotide regions encoding runs of compositionally simple amino acids subject to the mutational mechanism of replication slippage. Homoplasy at longer time scales manifests itself by incoherent distribution over a known phylogenetic tree. Convergent evolution can also be driven by selective advantage for altered length.
Indels occur very unevenly across the length of a given protein homology class. The rate might be high in terminal regions if the amino or carboxy termini are unimportant to the fold or function of matured protein. Within folded regions of soluble proteins, indels are greatly concentrated in loop regions of the 3D structure where a change in length can be accommodated without structural disruption. The distributional occurrence of indels even allows prediction of loop regions.
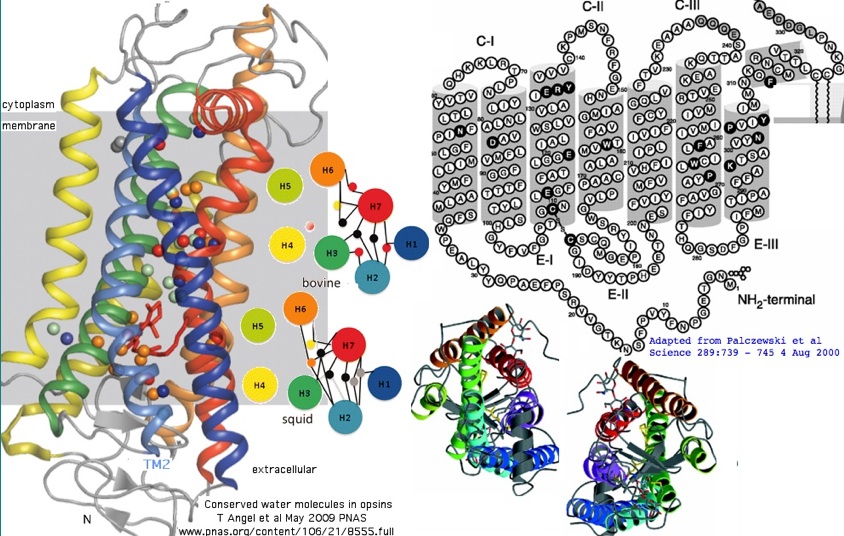
For integral membrane proteins such as GPCR, deletions are very rarely fixed in the transmembrane helical regions because a shortened length would no longer span the membrane at the same angle, thus pulling in inappropriate non-hydrophobic residues from soluble loops. Insertions too are rare because they push hydrophobic and boundary turn residues out into soluble compartments and distort connecting loops, perhaps altering insertion angles of adjacent transmembrane regions. Such mutations arise frequently enough but are rarely fixed at the population level or hang on as balanced alleles over timescales commensurate with ordinal speciations.
In massively expanded gene families such as GPCR, a coherently fixed indel in one descendent clade of the gene tree suggests adaptive sub- or neo-functionalization: if the indel were merely tolerated as near-neutral change, over geological timescales homoplasy at that site would occur. A remarkable site in transmembrane helix 2 was proposed in May 2009:
'Class A GPCR constitute a large family of transmembrane receptors. Helical distortions play a major role in the overall fold of these receptors. Most are related to conserved proline residues. However, in transmembrane helix 2, the proline pattern is not conserved, and when present, proline may be located at position TM 2.58, 2.59, or 2.60 yielding a bulged structure in P2.59 and P2.60 receptors or a more typical proline kink in P2.58 receptors. The proline pattern of helix 2 can be used as an evolutionary marker of molecular divergence of class A GPCRs.
At this site, two independent indel events occurred. One [unresolvable] indel arose very early in GPCR evolution in a bilateran ancestor before protostome-deuterostome divergence. This indel led to the split between the P2.58 somatostatin/opioid receptors and peptide receptors with the P2.59 pattern. Subfamilies with proline at position 2.59 or no proline expanded earlier, whereas P2.60 receptors remained marginal throughout evolution. P2.58 receptors underwent later rapid expansion in vertebrates with the development of the chemokine and purinergic receptor subfamilies from somatostatin/opioid-related ancestors. A second indel, resolvable as a deletion, occurred in insect melanopsins.'
This result refines the classification of Class A GPCR, which might be quite indecisive at certain gene tree nodes from sequence alignment alone. Timing of the insect deletion can be done better (below) because the SwissProt collection used by the authors carries only 20% of the melanopsins actually available. Note the structural significance of length and bulge changes can be examined in available 3D determinations. The functional effect of this shift in TM2 remains obscure but must be important.
Class Gene PDB Protein PubMed Best human opsin Next Best Signaling T.60.1 RHO1_bosTau 1JFP 3C9M 2J4Y bovine rod rhodopsin 17825322 RHO1_homSap 93% SWS1_homSap 45% Gt GNAT1 raises cGMP P.60.0 MEL1_todPac 2Z73 2ZIY squid melanopsin 18480818 MEL1_homSap 43% PER1_homSap 30% Gq GNAQ? inositol trisphosphate P.59.3 ADORA2A_homSap 3EML adenosine receptor 2A 18832607 MEL1_homSap 27% ENCEPH_homSap 27% Gs GNAT3 raises cAMP P.59.1 ADRB1_melGal 2VT4 beta 1 adrenergic receptor 18594507 MEL1_homSap 29% ENCEPH_homSap 25% Gs GNAT3 raises cAMP P.59.1 ADRB2_homSap 2R4R beta 2 adrenergic receptor 17962520 MEL1_homSap 28% PER1_homSap 29% Gs GNAT3 raises cAMP
Thus indels in opsins -- when they occur in a conserved region -- are potentially very informative as rare genetic events not appreciably subject to homoplasy in defining orthology classes and higher order clusterings of them, hopefully corroborating or even refining trees derived from sequence clustering by alignment. While precious, such data is limited because physiological and structural constraints have prevented most regions of opsins from ever accommodating an indel.
Indels in ciliary opsins
The tertiary structural integrity requirements of a 7-transmembrane opsin, along with tuned binding of retinal, isomerization cycle conformational shifts and binding to secondary protein contributers to the photoreception cycle, conspire to greatly constrain admissible locations for ciliary opsin indels. Indeed this varies greatly by region, with indels never seen in the transmembrane regions themselves (despite tens of billions of branch length years) and restricted in connecting cytoplasmic and extracellular loops to EC2 and IC3 and IC7. Indel incidence is much higher in amino and carboxy terminal tails but not useful because of gapping ambiguity issues.
The distribution of fixed indels is quite peculiar: almost all occur in gene family stems (ie shortly after gene duplication in one branch), hardly any occur mid-history. For vertebrate imaging opsins, this means prior to lamprey divergence. In other words, not only had all the classes of imaging opsins emerged post-tunicate/amphioxus pre-lamprey but (neglecting tails) also all their indels. No further indels arose in the subsequent 500 million years in any of these opsins, as if these opsins were already optimized from the length perspective
Consequently the rate of indel occurrence per billion years of branch length -- and so the frequency of multiple independent events near a given site -- is highly correlated to region, ie each region has a characteristic time scale over which it can be informative: too long and the risk of homoplasy (convergent evolution) is too high. That risk is exacerbated by uncertainty in gap placement within an alignment, which first requires delimitation by flanking invariant residues. Gap length per se is ambiguous: an indel of 3 residues shared by two extant species might have arisen once as a single event in the first species or as two events (one and two residues successively) in the other. Thus any phylogenetic interpretation of indels must be tempered by knowledge of the regional indel susceptibilities and the assumption these remain fairly constant across lineages and time.
Informative indels show up as readily apparent columns of gaps in large-scale alignments. If present across a single opsin orthology class, that merely validates prior blast clustering and other rare genomic events in establishing those classes in the first place. Sporadic indels, defined here as indels found within a single opsin gene, arise from sequencing errors but if not might be an adaptive specialization. It's very rare to see a ciliary opsin indel restricted to a phylogenetic subclade but examples exist: the post-marsupial loss of 5 residues of RHO1 in the distal arrestin binding region.
We're concerned here primarily with non-sporadic indels that span two or more orthology classes that speak to unresolved dating and topological issues in the gene tree. Significant individual indels visible on the alignment page. These give rise to a table sortable by position along the opsin sequence, indel length, region (eg 3rd cytoplasmic loop), higher taxonomic clade, and phylogenetic depth. Specific goals are dating indel events, characterizing remote opsins in pre-vertebrate deuterostomes, correctly placing cnidarians opsins, disambiguating opsins from non-opsin GPCR, and establishing ancestral lengths.
For deuterostome ciliary opsins, the story is fairly simple up to encephalopsin. None of the transmembrane helices have indels. That holds also for the first two cytoplasmic loops and first and last extracellular loops. Structural constraints can be too rigid, as illustrated by the well-known hydrogen bond chain of extremely conserved residues that holds the transmembrane helices in a fixed relative position: N55 in TM1 hydrogen bonded to D83 in TMH2 to peptide A299 in TMH6. Indels that altered the position of these residues within the respective helical wheels would cause the whole arrangement to become unglued. The asparagine and aspartate are deeply invariant not only in opsins but also GPCR.
The second extracellular loop has a two residue insert in all rod and cone opsins in a region so far not attributed functional significance; this may have been a near-neutral event in the ancestral stem protein (ie in a gene duplicate of pinopsin). The cytoplasmic side has all the protein-protein interactions but length of the extracellular loops can still be important in tensioning of transmembrane helices that sets their angles of insertion and relative orientation.
The third cytoplasmic loop has variable length distally. Length is constant within orthology classes with parietopsin having full length, parapinopsin one residue shorter, and all others two residues fewer. This is a region of high beta factor in bovine rhodopsin crystals, ie has too much movement to be assigned a conformation. Unsurprisingly no function has been assigned. While the indel pattern supports the conventional gene tree, evidently this indel hotspot has fixed at least three separate events. While that hasn't resulted in overt homoplasy in terms of length, additional events could be masked. This weakens interpretive certainty of indels in this region.
The amino terminus has 4 informative indels, all deletions. The first unites unites RHO1 and RHO2 to the exclusion of all other opsins (as does the short highly conserved N-terminus with two glycosylation sites). No indel or intron distinguishes them. RHO2 has an odd phylogenetic distribution -- it seems to occur in one species of lamprey but not in genomic lamprey (despite 19 million traces) nor in cartilaginous nor ray-finned fish, but seeming rises again in lungfish, coelacanth, lizards, and chicken but not frog nor any mammal. Possibly the lamprey RHO2 is a lineage-specific duplication of lamprey RHO1. A later independent duplication in lobe-finned fish persisted until the mammalian nocturnal loss era. It may be missing in frog because of an incomplete genome.
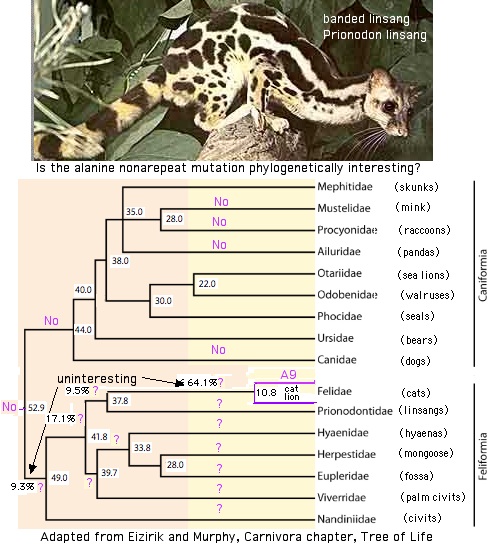
A restricted SWS1 indel partitions Carnivora
Caniformia-restricted deletion in SWS1 splits Caniformia (dogs, bears, seals...) off from Felidae (cats, civits ...) within Carnivora. Whales and dolphin SWS1 are all recent pseudogenes. Despite this, they are full length but all exhibit an unprecedented N --> D substitution at the N D P C iron triangle. This N is deeply conserved in almost all opsin and even GPCR. This was may have been adaptive in some way initially but set the stage for later pseudogenization.
SWS1_homSap LNAMVLVATLRYKKLRQPLNYILVNVSFG G FLLCIFSV F PVFVASCNGYFVFGRHVC human SWS1_tarSyr LNAMVLVATLHYRKLRQPLNYILVNVSLG G FLLCIFSV L PVFIASCRGYFVFGRHVC tarsier SWS1_oryCun LNAMVLVATLRYKKLRQPLNYILVNISLA G FLACIFSV F NVFVASCYGYFVFGRFVC rabbit SWS1_ratNor LNATVLVATLHYKKLRQPLNYILVNVSLG G FLFCIFSV F TVFIASCHGYFLFGRHVC rat SWS1_ailMel LNATVLVATLRYRKLRQPLNYILVNVSLA G FVYCI-SV S TVFIASCHGYFIFGRHVC panda SWS1_canFam LNGTVLVATLRYKKLRQPLNYILVNVSLG G FLYCI-SV S TVFIASCQGYFVFGRHVC dog SWS1_enhLut LNATVLVATLRYKKLRQPLNYILVNVSLG G FIYCI-SV S SVFIASCHGYFIFGHHIC otter SWS1_phoVit LNASVLVATLRYKKLRQPLNYILVNVSLG G FLYCI-SV S SVFIASCQGYFIFGRHVC seal SWS1_ursMar LNATVLVATLRYRKLRQPLNYILVNVSLA G FVYCI-SV S TVFIASCHGYFIFGRHVC bear SWS1_felCat LNATVLVATLRYRKLRQPLNYILVNVSLG G FLYCVSSV S IVFITSCHAYFIFGRHVC cat SWS1_hipAmp LNATVLVATLRYRKLRQPLNYILVNVSLG G FIYCIFSV F VVFITSCHGYFVFGRHVC hippo SWS1_ptePum LNATVLVATLRYRKLRQPLNYILVNVSLG G FLFCIFSV F TVFIASCQGYFVFGRHVC bat SWS1_talEur LNATVLVATLRYRKLRQPLNYILVNVSLG G FLFCIFSV L TVFIASCKGYFIFGRHVC mole SWS1_sorAra LNATVLVPTLRYRKLRQPLNYILVNVSLG G FLFCIFSV F TVIIASCKGYFVIGRHVC shrew SWS1_susScr LNATVLVATLRYRKLRQPLNYILVNVSLG G FIYCIFSV F SVFIASCHGYFVFGRRVC pig SWS1_bosTau LNATVLVATLRYRKLRQPLNYILVNVSLG G FIYCIFSV F IVFITSCYGYFVFGRHVC cow SWS1_lamPac LNATVLIATLRYRKLRQPLNYILVNVSLG G FIYCMFSV F CVFVASCYGYFVFGRRVC lama SWS1_turTru LDATVLVATLRYRKLRQPLNYILVNVSLG G FIYCIFSV F VVFITSCHGYFVFGRHVC dolphin SWS1_echTel LNAVVLVATLRYRKLRQPLNYILVNVSLA S VLFCVISV F TVFVASCHGYFIFGRHVC hyrax SWS1_monDom LNAVVLVATLRYKKLRQPLNYILVNVSLC G FIFCIFAV F TVFISSSQGYFIFGRHVC SWS1_smiCri LNGVVLIATLRYKKLRQPLNYILVNISLA G FIFCVFSV F TVFVSSSQGYFVFGRHVC SWS1_tarRom LNAVVLIATLRYKKLRQPLNYILVNISLA G FIFCVISV F TVFISSSQGYFIFGRHVC SWS1_galGal LNAVVLWVTVRYKRLRQPLNYILVNISAS G FVSCVLSV F VVFVASARGYFVFGKRVC SWS1_taeGut LNAIVLIVTIKYKKLRQPLNYILVNISVS G LMCCVFCI F TVFIASSQGYFVFGKHMC SWS1_anoCar LNAIILIVTVKYKKLRQPLNYILVNISFA G FLFCTFSV F TVFMASSQGYFFFGRHVC SWS1_utaSta LNAIILIVTVKYKKLRQPLNYILVNISFA G FLFCVFSV F TVFLASSQGYFFFGRHIC SWS1_xenLae LNFIVLLVTIKYKKLRQPLNYILVNITVG G FLMCIFSI F PVFVSSSQGYFFFGRIAC SWS1_neoFor LNAIVLFVTIKYKKLQQPLNYILVNISLA G FIFCFFGV F AVFIASCQGYFIFGKTVC SWS1_danRer MNGIVLFVTMKYKKLRQPLNYILVNISLA G FIFDTFSV S QVSVCAARGYYSLGYTLC SWS1_oryLat LNFVVLLATAKYKKLRVPLNYILVNITFA G FIFVTFSV S QVFLASVRGYYFFGQTLC SWS1_petMar LNAIVLIVTVKCKKLRQPLTYMLVNISAA G LVFCLFSI S TVFLFSTQGYFVFGPTVC SWS1_geoAus LNAIVLVVTIKYKKLRQPLNYILVNISAA G LVFCLFSI S TVFVASMQGYFFLGPTIC
Indels in melanopsins: TM2 region
The mid-transmembrane helix region preceding the proline in TM2 -- the only opsin transmembrane helix ever to experience an indel in 100 billion years of branch length evolution -- exhibits various independent insertions and deletions. That would seem to undercut efforts to make the length a definitive fundamental classifying tool among GPCR. The situation can be compounded by separate indels following the proline that, depending on gap placement, might affect the extracellular loop connecting TM2 and TM3.
However with care, the homoplasy is manageable, making the locus is quite informative for opsins (though a detailed analysis is necessary to fully exploit it).
An 'iron triangle' provides a fixed upstream frame of reference critical to reliable gapping of indels in this region. This consists of a very conserved Asn55 in TM1 hydrogen bonded to the almost universal charged residue Asp83 internal to TM2 which is further hydrogen bonded via internal H20 to N of the terminal NPXXY motif and a peptide amide Ala299 in TM7 (bovine rhodopsin numbering). The iron triangle is central to the proper associative bundling and relative orientation of the seven transmembrane helices in the vicinity of the Schiff base K296. No indels occur in any opsin or GPCR between this N and D (meaning cytoplasmic loop CL1 is of fixed length, namely 12 aa). Note from the full alignment that D83 has been replaced by G in all teleost fish RHO2 and all SWS1; it is mixed with N83 in some RHO1, RHO2 and entirely N83 in SWS2 but ancestrally strictly D in basal ciliary opsins.
Downstream, the reference frame is augmented by the first cysteine C110 of the universal GPCR disulfide linking TM3 to EC2. This is preceded by an easily recognized ancient motif WIFG (squid melanopsin; human G106R causes retinitis pigmentosa), which forces all gaps to be placed between the iron triangle D, the proline P and WIFGFAAC (FVFGPTGC in bovine rhodopsin). Thus post-proline gapping is quite constrained by reliable anchors.
Proline, as an imino acid incapable of alpha helix participation, plays a special role in GPCR transmembrane helices, kinking or bulging them. Shifting the position of a proline one residue forward or back relative to the 3.4 residues per turn helical wheel (view down axis) alters both the angle of resumption of the helix and its membrane-exiting residue position, perhaps somewhat torquing the connection to the following transmembrane helix TM3.
The effect is not dramatic in terms of angstroms of shift (as can be seen from a recent 3D alignment of helix TM2 that compares bovine and squid opsins, yet it follows from comparative genomics that the consequences for adsorption spectrum and/or regulation of signaling must be substantial. In other words, gene clade specific retention of proline or specific substituents observed in the massive alignment below holding for billions of years of branch length is only feasible when adaptive.
The 185 ciliary opsins (which includes 5 basal cnidarian opsins) in the reference sequence collection are all of the same length in this region (except for odd Apis and Platynereis sequences), as are 65 peropsins, RGR and neuropsins, many melanopsins, and the vast majority of near-opsin GPCR. Consequently this length, denoted P59.2 (for proline in position 59 bovine rhodopsin numbering and 2 residues shorter in the proline-cysteine region than the longest opsins, is ancestral for melanopsins which themselves vary in length.
Deuterostome melanopsins are all of P.59.2 type, as are LMS and BCR arthropod melanopsins, a subclass of lophotrochozoan melanopsins, and the one known cnidarian melanopsin. The remaining dozen known lophotrochozoan melanopsins are all type P.60.2. This class -- which fortunately includes the structurally determined squid melanopsin -- thus has a one residue insertion whose location appears to be 5 residues after the D and 4 before the P.
Thus lophotrochozoan melanopsins had ancestral length up to a gene duplication which subsequently acquired this stem insertion in a descendent copy. A single other human GPCR, namely thyrotropin-releasing hormone receptor TRHR, is also P.60.2, demonstrating homoplasy. However given the rarity of transmembrane indel events, the history here can be reliably disambiguated assuming parsimony.
The three classes of ecdysozoan ultraviolet melanopsins (represented by 44 genes) all share a one residue deletion in this same region, approximately at the 4th post-D residue, making them P.58.2 class, homoplasic to within gap placement to moderately abundant GPCR (eg somatostatin receptor). This event, affecting insects, crustaceans and chelicerates, occurred deep within the stem lineage of ecdysozoa. More data from early diverging arthropods is needed to refine the timing. Recall these opsins have a peculiar lysine K90 (sometimes E90) that tunes their adsorption into the ultraviolet. The extra residue loss may be required to correctly position the K90 for its blueshift.
The three molluscan melanopsins of ancestral length share a striking signature aspartate residue two position preceding the proline, ie at this same K90 position. (Recall G90D and T94I in human RHO1 constitutively activate transducin in absence of chromophore and cause night blindness.) Consequently these three opsins may also have their adsorption shifted towards the UV since otherwise G90 is present in lophotrochozoan melanopsins. They should be renamed (ie reclassified) to reflect probable parental character, with P.60.2 lophotrochozoan opsins renamed to MEL2.
The post-proline pre-cysteine region has length variations that represent insertions in various homology classes. They are difficult to gap reliably other than occurring at the distal end of TM2 before the conserved block of extracellular loop EL1. As TM2 (by definition) just reaches the surface, these extra residues can be attributed to lengthened EL1. It emerges that indels outside the D to P region are only moderately informative. They may suffice to define narrow classes of opsins where blast clustering is ambiguous. While pseudo-homoplasic, that is readily resolvable given the sequence cluster isolation:
- Three amphioxus melanopsins (eg MEL6_braFlo) have a 1 residue distal deletion but MELmop_braFlo does not. This event constitutes an isolated class of sequences.
- Nine melanopsins from Branchiopoda have a 1 residue distal insertion. Three other melanopsins from this group have a further 1 residue insertion. This group of melanopsins has other odd properties; these could possibly have deeper ancestral roots but data is lacking from earlier branching arthropods.
- RGR opsins all have a 1 residue distal deletion; however two Ciona opsins have seemingly regained a residue. Five Ciona RGR have a deletion preceding the D. However because the proline anchor is lacking, placement is otherwise uncertain in this isolated opsin class. These same five opsins are unique in having tyrosine in place of the conserved asparagine N in TM1 (that bonds to D).
- Five peropsins have an inserted residue preceding the D. This appears to define PER2 opsins which are currently restricted to amphioxus and sea urchin. Hemichordates have a peropsin of type PER1 lacking the insert. Lophotrochozoan peropsins also lack it. Thus it appears to be a very restricted early gene expansion that did not persist in vertebrates.
- NEUR4 neuropsins have a large distal insertion of 4 residues. This class of opsins is quite obscure and lacks the proline.
Departures from the conserved N D P C format are uncommon. RGR is Y/N D VMITAL C and NEUR 4 neuropsins are consistently N D S C. Ciliary opsins are the only major group departing from this pattern. Most provocatively, the very earliest TMT opsins from deuterostomes, ecdysozoa and cnidaria have the standard pattern, establishing it as unquestionably ancestral for ciliary opsins.
These opsins should be renamed to reflect this classificatory principle because they provide the ciliary ur-opsin form and quite possibly function. They cannot be successfully modeled in TM2 using bovine rhodopsin structure because it lacks the proline and its induced kink.
Using known fish ciliary ur-opsins as probes and the N D P C (especially P) as extra criterion, it emerges that both frog and lizard have a ciliary ur-opsin in syntenic location. However chicken, platypus, marsupial, and placental mammal do not. Gene order is preserved in chicken but no pseudogene remains at this site. This is a familiar story in opsins ... an old gene fades out mid-amniote but otherwise continues on 310 million year (Wall hypothesis).
No transcript data or reference gene information is available for frog or lizard ciliary ur-opsin , meaning nothing is known about site of expression. However this opsin has been specifically studied in fish, amphioxus and sea urchin. Testis is one site of expression.
Alignment of TM2 proline region in lophotrochozoan melanopsins with included representative outgroup sequences. Numbers in parentheses indicate total number of reference sequences represented by the proxy sequence: MEL1_todPac GNGIVIYLFTKTKSLQTPANMFIINLAFSDFTFSLVNGFPLMTISCFLKKWIFGFAAC P.60.2 (1) MEL1_sepOff GNGIVIYLFTKTKSLQTPANMFIINLAFSDFTFSLVNGFPLMTISCFIKKWVFGMAAC P.60.2 (1) MEL1_entDof GNGVVIYLFSKTKSLQTPANMFIINLAMSDLSFSAINGFPLKTISAFMKKWIFGKVAC P.60.2 (1) MEL1_patYes GNTTVVYIFSNTKSLRSPSNLFVVNLAVSDLIFSAVNGFPLLTVSSFHQKWIFGSLFC P.60.2 (1) MEL1_lotGit GNFVVIYTFSRTKSLRTASNMFVVNLALSDLTFSAVNGFPLFSLSSFSHKWIFGRVAC P.60.2 (1) MEL1_plaDum GNLLVVWTFLKTKSLRTAPNMLLVNLAIGDMAFSAINGFPLLTISSINKRWVWGKLWc P.60.2 (1) MEL1_schMed GNLLVLYIFARAKSLRTPPNMFIMSLAIGDLTFSAVNGFPLLTISSFNTRWAWGKLTC P.60.2 (1) MEL1_capCap GNLVVITLFIKTRSLRTPPNMFIINLALSDMGFCATNGFPLMTVASFQKLWRWGPVAC P.60.2 (1) MEL1_schMan GNSLVITLFLLCKQLRTPPNMLIVSLAISDFSFALINGFPLKTIAAFNHRWGWGKLAC P.60.2 (1) MEL2_schMan LNLLVIVFFTMFKSLRTPSNILVVNLAISDFGFSAVIGFPLKTMAAFNNFWPWGKLAC P.60.2 (1) MEL3_schMan TNLLVIFVFLTPKSSISLQCALIINLAISDFGFSAVIGFPLKTIAAFNQYWPWGSVAC P.60.2 (1) MEL1_helRob GNIIVVWVFSRTPSLRTPSNVLVINLAICDILFSALIGFPMSALSCFQRHWIWGNFYC P.60.2 (1) MEL2_helRob TPILRTHANVLIINLALCDLIFSSLIGFPMTALSCFKRHWIWGDLGC P.60.2 (1) MEL1_aplCal GNSLVIITCIRFKDLRTRSNILIINLAVGDLLMC-LIDFPLLAAASFYGEWPYGRQVC P.59.2 (1) MEL2_lotGig GNSIVIWAHVRIKSLSTTSNMLILNLCVGCLIMC-IVDFPLYATSSFLQKWIFGHKVC P.59.2 (1) MEL2_aplCal RHSSLRTSSNLLVVNLTVADLVMS-SLDFPILAISSYKGCWVMGFLGC P.59.2 (1) LMS1_droMel GNGVVIYIFATTKSLRTPANLLVINLAISDFGIM-ITNTPMMGINLYFETWVLGPMMC P.59.2 (23) MEL1_homSap GNLTVIYTFCRSRSLRTPANMFIINLAVSDFLMS-FTQAPVFFTSSLYKQWLFGETGC P.59.2 (20) MEL2_strPur GNSLVIYTFLRFKKLHSPINLLIVNLSASDLLVA-TTGTPLSMVSSFYGRWLFGTNAC P.59.2 (10) TMTa1_danRe NNLLVLVLFGRYKVLRSPINFLLVNICLSDLLVC-VLGTPFSFAASTQGRWLIGDTGC P.59.2 (185) PER1_homSap SNIIVLGIFIKYKELRTPTNAIIINLAVTDIGVS-SIGYPMSAASDLYGSWKFGYAGC P.59.2 (33) NEUR1_homSa GNGYVLYMSSRRKKKLRPAEIMTINLAVCDLGIS-VVGKPFTIISCFCHRWVFGWIGC P.59.2 (30) UV7_droMel GNAFVIFMFANRKSLRTPANILVMNLAICDFLM--LIKCPIAIYNNIKEGPALGDIAC P.58.2 (14) UV5_apiMel GNGLVIWIFCAAKSLRTPSNMFVVNLAICDFFM--MIKTPIFIYNSFNTGFALGNLGC P.58.2 (20) UVB_nasVit GNGCVVWIFSTSKVLRTPSNLFIINLALFDLVM--ALEIPMLIINSFIERMIGWGLGC P.58.2 (8)
Alignment in TM2 region: 420 curated opsins
Colored blocks show useful opsin gene tree synapomorphies -- derived states relative to last common ancestor. The TM2 region is so rich in these that it can resolve many difficult gene classification issue and thus might be called the Rosetta Stone region of opsins -- 10 are highlighted below. Genes altered by an indel are colored magenta) -- ie the indel was resolvable as a specific insertion or deletion relative to ancestral. Note the consistency of blocks with gene names derived independently via blast clustering without consideration of introns, indels, and other rare genomic events.
Some phyoSNPs at key amino acids are also shown in red, notably the K90 of insect ultraviolet melanopsins and sole class of ciliary opsins with ancestral proline surviving at position 59 which distinguishes the ciliary ur-opsin class TMTa from later gene duplications that became other TMT homologs and encephalopsins. SWS1 opsins all have an asparagine in place of the key aspartate; RHO2 in teleost fish all have a glycine; NEUR4 all have a serine here as well as a 4 residue insert.
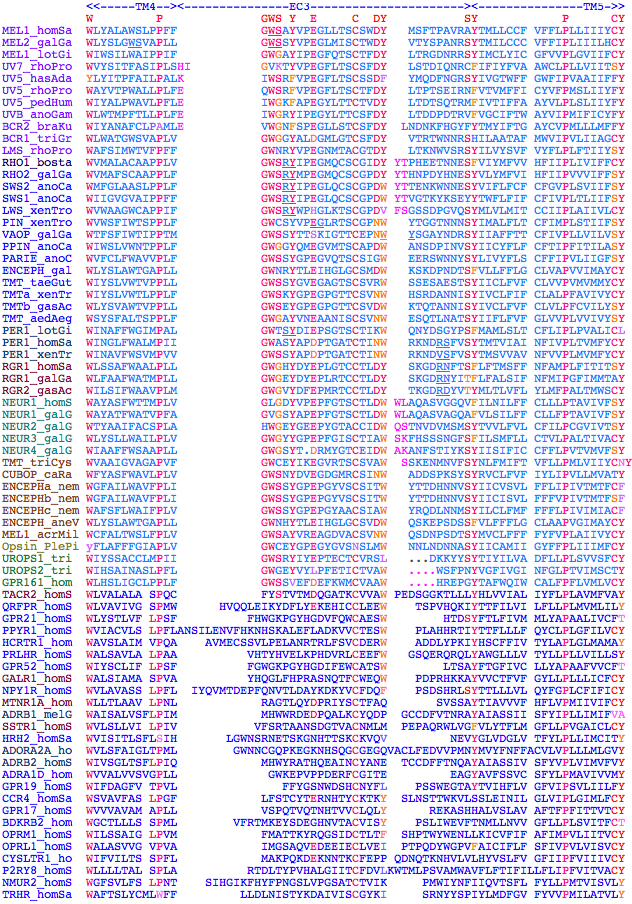
--TM1-----><---CL1---><---------------TM2------------><--EC1---><-TM3 MEL1_todPa G N GIVIYLFTKTKSLQTPANMFIINLAFS D FTFSLVNGF P LMTISCFL-- KKWIFGF AA C P.60.2 MEL1_sepOf G N GIVIYLFTKTKSLQTPANMFIINLAFS D FTFSLVNGF P LMTISCFI-- KKWVFGM AA C P.60.2 MEL1_entDo G N GVVIYLFSKTKSLQTPANMFIINLAMS D LSFSAINGF P LKTISAFM-- KKWIFGK VA C P.60.2 MEL1_patYe G N TTVVYIFSNTKSLRSPSNLFVVNLAVS D LIFSAVNGF P LLTVSSFH-- QKWIFGS LF C P.60.2 MEL1_lotGi G N FVVIYTFSRTKSLRTASNMFVVNLALS D LTFSAVNGF P LFSLSSFS-- HKWIFGR VA C P.60.2 MEL1_plaDu G N LLVVWTFLKTKSLRTAPNMLLVNLAIG D MAFSAINGF P LLTISSIN-- KRWVWGK LW C P.60.2 MEL1_schMe G N LLVLYIFARAKSLRTPPNMFIMSLAIG D LTFSAVNGF P LLTISSFN-- TRWAWGK LT C P.60.2 MEL1_capCa G N LVVITLFIKTRSLRTPPNMFIINLALS D MGFCATNGF P LMTVASFQ-- KLWRWGP VA C P.60.2 MEL1_schMa G N SLVITLFLLCKQLRTPPNMLIVSLAIS D FSFALINGF P LKTIAAFN-- HRWGWGK LA C P.60.2 MEL2_schMa L N LLVIVFFTMFKSLRTPSNILVVNLAIS D FGFSAVIGF P LKTMAAFN-- NFWPWGK LA C P.60.2 MEL3_schMa T N LLVIFVFLTPKSSISLQCALIINLAIS D FGFSAVIGF P LKTIAAFN-- QYWPWGS VA C P.60.2 MEL1_helRo G N IIVVWVFSRTPSLRTPSNVLVINLAIC D ILFSALIGF P MSALSCFQ-- RHWIWGN FY C P.60.2 MEL2_helRo . . .........TPILRTHANVLIINLALC D LIFSSLIGF P MTALSCFK-- RHWIWGD LG C P.60.2 MEL1_aplCa G N SLVIITCIRFKDLRTRSNILIINLAVG D LLMC-LIDF P LLAAASFY-- GEWPYGR QV C P.59.2 MEL2_lotGi G N SIVIWAHVRIKSLSTTSNMLILNLCVG C LIMC-IVDF P LYATSSFL-- QKWIFGH KV C P.59.2 MEL2_aplCa . . ........RHSSLRTSSNLLVVNLTVA D LVMS-SLDF P ILAISSYK-- GCWVMGF LG C P.59.2 MEL1_dapPu A N STILYVFSRFKRLRTPANVFIINLTIC D FLA--CCLH P LAVYSAFR-- GRWSFGQ TG C P.58.2 MEL1_homSa G N LTVIYTFCRSRSLRTPANMFIINLAVS D FLMS-FTQA P VFFTSSLY-- KQWLFGE TG C P.59.2 MEL1_felCa G N LMVIYTFCRSRGLRTPANMFIINLAVS D FFMS-FTQA P VFFASSLH-- KRWLFGE AG C P.59.2 MEL1_ailMe G N LMVIYTFCRTRGLRTPSNMFIINLAVS D FLMS-FTQA P VFFASSLH-- KRWLFGE AG C P.59.2 MEL1_canFa G N LMVIYTFCRTRGLRTPSNMFIINLAVS D FFMS-FTQA P VFFASSLH-- KRWLFGE AG C P.59.2 MEL1_myoLu G N LTVIYTFCRSRGLRTPANMFIINLAVS D FLMC-FTQA P VVFASSIY-- KRWLFGE AG C P.59.2 MEL1_pteVa G N LTVIYTFCRSRGLRTPANMFIINLAVS D FLMS-FTQA P VVFISSLY-- KRWLFGQ AG C P.59.2 MEL1_smiCr G N LLVIYTFCRSRSLRTPANMFIINLAIS D FFMS-FTQA P VFFASSLY-- ERWIFGE KG C P.59.2 MEL1_monDo G N FLVIYTFCRSHSLRTPANMFIINLAIS D FFMS-FTQA P VFFASSMY-- KRWIFGE KA C P.59.2 MEL1_loxAf G N LMVIYIFFRSRGLRTPANMFIINLAVS D FLMS-FTQA P VFFASSLY-- KRWLFGE AG C P.59.2 MEL1_taeGu G N FLVFYAFCRSRSLQTPANILIINLAIS D FLMS-ITQS P VFFTSSLY-- KHWIFGE KG C P.59.2 MEL1_galGa G N FLVIYAFCRSRTLQKPANIFIINLAVS D FLMS-ITQS P VFFTNSLH-- KRWIFGE KG C P.59.2 MEL1_xenTr G N FLVIYAFCRSRSLRSPANMFIINLAIT D FLMS-VTQA P VFFATSLH-- KRWIFGE KG C P.59.2 MEL1_danRe G N FLVIYAFSRSRTLRTPANLFIINLAIT D FLMC-ATQA P IFFTTSMH-- KRWIFGE KG C P.59.2 MEL1_takRu G N FLVIYAFCRSRSLRTPANMFIINLAVT D LLMC-VTQT P IFFTTSMY-- KRWIFGE KG C P.59.2 MEL1_gasAc G N VLVIYAFSKSRSLRTPANMFIINLAIT D LLMC-VTQA P IFFTTSMH-- KRWIFGE KG C P.59.2 MEL1_oryLa G N FLVIYAFSRSRSLRTPANMFIINLAIT D LLMC-VTQS P IFFTTSMH-- KRWIFGE KG C P.59.2 MEL1_calMi G N FLVIYAFLRSRSLRTPANTFIINLAAT D FLMS-VTQS P IFFITSIH-- KRWIFGE KG C P.59.2 MEL1_petMa G N VLVIYAFSKSKSLRSPANIFIINLAFA D FFMS-ITQT P IFFVTSLH-- KRWIFGE KG C P.59.2 MELmop_bra G N AVVVYSFIKSKGLRTPANFFIINLALS D FLMN-LTNM P IFAVNSAF-- QRWLLSD FA C P.59.2 MELmop_bra G N AVVVYSFIKSKGLRTPANFFIINLALS D FLMN-LTNM P IFAVNSAF-- QRWLLSD FA C P.59.2 MEL1_strPu . . ........WTKSLRTPPNMLIVNLAIS D FGMV-ITNF P LMFASTIY-- NRWLFGD AG C P.59.2 MEL2_strPu G N SLVIYTFLRFKKLHSPINLLIVNLSAS D LLVA-TTGT P LSMVSSFY-- GRWLFGT NA C P.59.2 MEL2_galGa G N LLVLYAFYSNKKLRTPQNFFIMNLAVS D FLMS-ASQA P ICFVNSLH-- REWILGD IG C P.59.2 MEL2_xenLa G N MLVLYAFYRNKKLRTAPNYFIINLAIS D FLMS-ATQA P VCFLSSLH-- REWILGD IG C P.59.2 MEL2_anoCa G N LLVLYAFYSNKRLRTPPNYFIMNLAVS D FLMS-ATQA P ICFLNSMH-- KEWVLGD IG C P.59.2 MEL2_tetNi G N VLVIFAFYSNKKLRSLPNYFIVNLAVS D LLMA-STQS P IFFIN-LY-- KEWMFGE TA C P.59.2 MEL2_danRe G N ALVMFAFYRNKKLRSLPNYFIMNLAVS D FLMA-ITQS P IFFINCLY-- KEWMFGE LG C P.59.2 MEL2_gasAc G N ALVMLAVYSNKKLRNLPNYFIMNLAVS D FLMA-FTQS P IFFINCLY-- KEWAFGE TG C P.59.2 MEL6_braFl G N AVALYAFCRSRSLRRPKNYLIANLCLT D MVVC-LVYS P IIVTRSL--- SHGLPSK ES C P.59.3 MEL6_braBe G N VVALYAFCRTRSLRRPKNYVVANLCLT D MFVC-LVYC P IVVSRSF--- SHGFPSK ES C P.59.3 MELx_braFl G N AVALYAFCSTRKLRRPKNYVVANLCLT D LIMC-IVYC P VIVISSF--- SGRIPTD GA C P.59.3 LMS1_droMe G N GVVIYIFATTKSLRTPANLLVINLAIS D FGIM-ITNT P MMGINLYF-- ETWVLGP MM C P.59.2 LMS2_droMe G N GVVVYIFGGTKSLRTPANLLVLNLAFS D FCMM-ASQS P VMIINFYY-- ETWVLGP LW C P.59.2 LMS6_droMe G N FIVMYIFTSSKGLRTPSNMFVVNLAFS D FMMM-FTMF P PVVLNGFY-- GTWIMGP FL C P.59.2 LMS_anoGam G N GMVIYIFSTAKSLRTPSNLFIVNLALS D FLMM-GTNA P TMVYNCWF-- ETWSLGL LM C P.59.2 LMS_rhoPro G N GMVIFIFSSTKTLRTPSNLLVVNLAFS D FLMM-FTMS P PMVINCYN-- ETWVLGP LM C P.59.2 LMS_schGre G N GMVIYIFSTTKSLRTPSNLLVVNLAFS D FLMM-FTMS A PMGINCYY-- ETWVLGP FM C P.59.2 LMS_lucCru G N GMVIYIFSTTKSLRSPSNLLVVNLAFS D FLMM-FTMA P PMVINCYN-- ETWVWGP LF C P.59.2 LMS_triCas G N GMVIYIFSSTKALRTPSNLLVVNLAFS D FLMM-LCMS P AMVINCYN-- ETWVLGP LV C P.59.2 LMS_manSex G N GMVIYIFMSTKSLKTPSNLLVVNLAFS D FLMM-CAMS P AMVVNCYY-- ETWVWGP FA C P.59.2 LMS_papXut G N GMVVYIFTSTKSLKTPSNLLVVNLAFS D FLMM-LCMA P PMLINCYY-- ETWVFGP LA C P.59.2 LMS_homCoa G N GMVVYIFSCTKALRTPSNLLVVNLAFS D FLMM-FTMA P PMVLNCYY-- ETWVLGP FM C P.59.2 LMSa_nasVi G N GMVVYIFASTKSLRTPSNLLVINLAFS D FCMM-FTMS P PMVINCYY-- ETWVFGP LM C P.59.2 LMSb_apiMe G N GMVVYIFLSTKSLRTPSNLFVINLAIS D FLMM-FCMS P PMVINCYY-- ETWVLGP LF C P.59.2 LMS_acyPis G N GMVIYIFTCTKNLRTPSNLLIVNLAFS D FCLM-FTMC P AMVWNCFY-- ETWMFGP FA C P.59.2 LMSb_nasVi G N GMVVYIFLVTPSLRTPSNLLVINLAFS D FVMM-IIMS P PMVVNCWY-- ETWILGP LM C P.59.2 LMSa_apiMe G N GVVVYVFIMTPSLRTPSNLLVVNLAFS D FIMM-GFMC P PMVICCFY-- ETWVLGS LM C P.59.2 LMS_meoOer G N FVVIWVFMNTKALRSPANTLVVSLAVS D FIMM-ACMF P PLVLNCYW-- GTWIFGP LF C P.59.2 LMS_limPol G N GMVIYLMMTTKSLRTPTNLLVVNLAFS D FCMM-AFMM P TMTSNCFA-- ETWILGP FM C P.59.2 LMS2_plePa G N GMVMYLMNTTKSLKTPTNMLIVNLAFS D FCMM-AFMM P TMAANCFA-- ETWILGP FM C P.59.2 LMS2_hasAd G N GMVIYLMSTTKSLKTPTNMLIVNLAFS D FCMM-AFMM P TMAANCFA-- ETWILGP LM C P.59.2 LMS_ixoSca G N SMVIYIMTTSKSLRSPTNMLVVNLAFS D WCMM-AFMM P TMAANCFA-- ETWILGP FM C P.59.2 LMS1_plePa G N SIVIYLMLSVKSLRTPANFLVTSLAVS D GGML-AFMA P TMPINCFA-- QTWVLGP FM C P.59.2 LMS1_hasAd G N GVVMYLMMTVKNLRTPGNFLVLNLALS D FGML-FFMM P TMSINCFA-- ETWVIGP FM C P.59.2 BCRa_hemSa G N GLVIYLYMKSQALKTPANMLIVNLALS D LIML-TTNF P PFCYNCFGS- GRWMFSG TY C P.59.1 BCRb_hemSa G N GLVIYLFNKSAALRTPANILVVNLALS D LIML-TTNV P FFTYNCFGS- GVWMFSP QY C P.59.1 BCR_porPel G N GMVIYLFAKCQALRTPANILVVNLALS D LIML-TTNV P FFTYNCFGN- GVWMFSA TY C P.59.1 BCR_triGra G N SLVISLFTKTKELRTPANMFVVNLAFS D LCMM-ITQF P MFVYNCFGN- GMWLFGP FL C P.59.1 BCR2_triLo G N SLVISLFTKTKELRTPANMFVVNLAFS D LCMM-ITQF P MFVYNCFGN- GMWLFGP FL C P.59.1 BCR_limPol G Q SVVLYLFAKTKPLRTPANMLIVNLAFS D FMMM-ITQF P VFIINCLGG- GAWQLGP LL C P.59.1 BCR2_braKu G N GLVIWIFLKTKSLRTPSNMLIVNLAIA D FFMM-LTQS P LYIISAFST- RWWIWGH FW C P.59.1 BCR3_braKu G N GLVIKIFLKTKSLRTPSNMLIVNLAIA D FFMM-LTQS P LFIISAFSS- RWWIWGH FW C P.59.1 BCR1_triGr G N YLVLRIFTKFQELRRPSNVLVINLALS D MLLM-LTLF P ECVYNFLGS- GPWRFGD LG C P.591 BCR2_triGr G N VLVLHIFGKHKNLRSPTNTLLMNLAFC D LMIF-IGLY P EMLGNIFMND GTWMWGD VA C P.59.0 BCR1_triLo G N VLVLHIFGKHKNLRSPTNTLLMNLAFC D LMIF-IGLY P EMLGNIFMND GTWMWGD IA C P.59.0 BCR3_triGr G N VLVLYIFGKYKSLRSPTNVLVMNLAFC D LGLF-VGLY P ELLGNIFINN GPWMWGD VA C P.59.0 UV7a_acyPi G N SLVIFMYFKCRSLQTPANMLIINLAVS D FIM--LAKA S VFIYNSYY-- LGPALGK LG C P.58.2 UV7b_acyPi G N SLVIFMYIKCKSLQTPANVLIMNLAVS D FIM--LAKT P VFIYNSFY-- QGPTLGK LG C P.58.2 UV7_rhoPro G N LLVIFMILRFRTLRTSSNILILNLAVS D FLM--VAKM P VFIYNSFY-- FGPVLGE MG C P.58.2 UV7_anoGam G N ALVVFMFYRYRSLRTPANYLVINLAVA D FII--MMEA P MFIYNSIH-- QGPALGS IG C P.58.2 UV7_aedAeg G N LLVILMFFRFKSLRTPANYLVINLAIA D FII--MLEA P LFVYNSYH-- QGPATGN VW C P.58.2 UV7_culQui G N VLVIFMFFKFKSLRTPANYLVINLAVA D FLI--MLEA P IFVYNSYH-- LGPAFGN TL C P.58.2 UV7_droMel G N AFVIFMFANRKSLRTPANILVMNLAIC D FLM--LIKC P IAIYNNIK-- EGPALGD IA C P.58.2 UV7_droYak G N AFVIFMFANRKSLRTPANILVMNLAIC D FLM--LIKC P IAIYNNIK-- EGPALGD IA C P.58.2 UV7_droAna G N AFVIFMFANRKSLRTPANILVMNLAIC D FLM--LVKC P IAIYNNIK-- EGPALGD VA C P.58.2 UV7_droPse G N AFVIFMFANRKSLRTPANILVMNLAIC D FLM--LVKC P IAIYNNIK-- EGPALGD AA C P.58.2 UV7_droWil G N AFVIFMFSNRKSLRTPANILVMNLAIC D FLM--LVKC P IAIYNNIK-- EGPALGD IA C P.58.2 UV7_droMoj G N AFVIFMFGSRKSLRTPANILVMNLAIC D FLM--LVKC P IAIYNNIQ-- EGPALGD AA C P.58.2 UV7_pedHum G N FLIIYLFLRKRSLRTPSNVFIFNLAVS D SLL--LLKM P VFIINSFY-- LGPALGN LG C P.58.2 UV7_ixoSca . . .........RRRIRSQANLLVFNLALS D LLM--VLEI P LLVYNSLK-- LRPALGV WG C P.58.2 UV5_plePay G N AIVMYIFFSAKTLRTPTNMFVIGLAMA D LLM--MSKT P VFIYNCFH-- LGPVFGQ IG C P.58.2 UV5_hasAda G N AIVIYIFSVSKSLRTPTNMFVIGLAMA D LLM--MSKT P VFIYNCFH-- LGPVFGQ LG C P.58.2 UV5_braKug G N GVVIWVFASAKSLRTPSNLFVINLAVL D FLM--MLKT P VFIVNSFN-- EGPIWGK TG C P.58.2 UV5_triLon G N GVVIWIFSSAKSLRTPSNMFVINLAVL D FIM--MMKT P VFIVNSFN-- EGPIWGK FG C P.58.2 UV5_triGra G N GVVIWIFSSAKSLRTPSNMFVINLAVL D FIM--MMKT P VFIVNSFN-- EGPIWGK FG C P.58.2 UV5a_dapPu G N GVVIWIFTNCKSLRTPSNMLVVNLAIL D MLM--MLKS P VMIINSYN-- EGPIWGK LG C P.58.2 UV5b_dapPu G N GIVIYIFSTTKELKTPSNILILNLAIC D FIM--MIKT P IFIVNSFN-- EGPVFGR LG C P.58.2 UV5_papXut G N GLVIFIFSASKSLRTPSNLLVVQLAVL D FLM--MLKA P IFIYNSIK-- RGFASGV IG C P.58.2 UV5_manSex G N GMVIFIFSTTKSLRTSSNFLVLNLAIL D FIM--MAKA P -FIYNSAM-- RGFAVGT VG C P.58.2 UV5_apiMel G N GLVIWIFCAAKSLRTPSNMFVVNLAIC D FFM--MIKT P IFIYNSFN-- TGFALGN LG C P.58.2 UV5_nasVit G N GLVIWIFCAAKSLRTPSNMFVVNLAIC D FMM--MLKT P IFIYNSFH-- TGFALGN LG C P.58.2 UV5_diaNig G N GLVIWVFSSAKTLRTPSNIFVINLALY D FIM--MLKT P IFIYNSFN-- LGFGLGQ LG C P.58.2 UV5_lucCru G N GLVLWIFSTSKSLKTASNMFVVNLAFC D FIM--MMKM P IFVYNSFN-- RGYALGH IG C P.58.2 UV5_triCas G N GLVIWIFSTSKSLRTASNMFVVNLAIC D FAM--MIKT P IFIYNSFY-- RGFALGH LG C P.58.2 UV5_anoGam G N GLVIWIFIAAKSLRTPSNVFVINLAIC D FFM--MAKT P IFIYNSFT-- KGFTLGN LG C P.58.2 UV4_droMel G N GMVIWIFSTSKSLRTPSNMFVLNLAVF D LIM--CLKA P IFIYNSFH-- RGFALGN TW C P.58.2 UV3_droMel G N GLVIWVFSAAKSLRTPSNILVINLAFC D FMM--MVKT P IFIYNSFH-- QGYALGH LG C P.58.2 UV5_rhoPro G N GLVIWIFSTAKTLRTPSNIFVVNLAIC D FLM--MSKT P IFIYNSFK-- LGYALGH RA C P.58.2 UV5_pedHum G N GIVIWIFTTSKNLRTASNVFVVNLAIF D FIM--MAKT P IMIYNSMN-- LGFECGF VW C P.58.2 UV5_acyPis G N GLVIWVFCVAKPLRTPSNIFVINLALC D FVM--MAKA P IFILGSIN-- RGY-QGH FL C P.58.2 UVB_anoGam G N GIVLWIFGTSKSLRNGSNMFIINLAIF D LLM--MCEM P MFLVNSFS-- ERLVGYG VG C P.58.2 UVB_diaNig G N GIVLWIFATTKSLRTPSNMFVVNQALL D LLM--MIEM P MFVLNSLYF- QRPIGWE MG C P.58.1 UVB_manSex G N GIVIWIFSTSKSLRSASNMFVINLAVF D LMM--MLEM P LLIMNSFY-- QRLVGYQ LG C P.58.2 UVB_apiMel G N CCVIWIFSTSKSLRTPSNMFIVSLAIF D IIM--AFEM P MLVISSFM-- ERMIGWE IG C P.58.2 UVB_nasVit G N GCVVWIFSTSKVLRTPSNLFIINLALF D LVM--ALEI P MLIINSFI-- ERMIGWG LG C P.58.2 UV5B_droMe G N GLVIWIFSTSKSLRTPSNLLILNLAIF D LFM--CTNM P HYLINATV-- GYIVGGD LG C P.58.2 UVB_acyPis G N GLVLWIFCVSKPLRTPSNLFVLNLALC D FSM--VLVL P ILIYDSID-- HKY-PGH LQ C P.58.2 UVB_megVic G N GLVLWIFCVSKPLRTPSNLFVLNLALC D FSM--VLVL P ILIYDSID-- HKY-PGH LQ C P.58.2 RHO1_bosTa I N FLTLYVTVQHKKLRTPLNYILLNLAVA D LFMV-FGGF T TTLYTSLH-- GYFVFGP TG C P.59.2 RHO1_homSa I N FLTLYVTVQHKKLRTPLNYILLNLAVA D LFMV-LGGF T STLYTSLH-- GYFVFGP TG C P.581 RHO1_monDo I N FLTLYVTIQHKKLRTPLNYILLNLAIA D LFMV-FGGF T MTLYTSLH-- GYFVFGP TG C P.59.2 RHO1_ornAn I N FLTLYVTIQHKKLRTPLNYILLNLAFA N HFMV-LGGF T TTLYTSLH-- GYFVFGP TG C P.59.2 RHO1_galGa V N FLTLYVTIQHKKLRTPLNYILLNLVVA D LFMV-FGGF T TTMYTSMN-- GYFVFGV TG C P.59.2 RHO1_anoCa I N FLTLFVTIQHKKLRTPLNYILLNLAVA N LFMV-LMGF T TTMYTSMN-- GYFIFGT VG C P.59.2 RHO1_xenTr I N FMTLYVTIQHKKLRTPLNYILLNLVFA N HFMV-LCGF T VTMYTSMH-- GYFIFGQ TG C P.59.2 RHO1_neoFo I N FLTLYVTVQHKKLRTPLNYILLNLAVA D LFMV-FGGF T TTMYTAMN-- GYFVFGV VG C P.59.2 RHO1_latCh I N FLTLFVTIQHKKLRTPLNYILLDLAVA D LCMV-FGGF F VTMYSSMN-- GYFVLGP TG C P.59.2 RHO1_angAn V N FLTLYVTIEHKKLRTPLNYILLNLAVA N LFMV-FGGF T TTVYTSMH-- GYFVFGE TG C P.59.2 RHO1_conMy I N FLTLYVTIEHKKLRTPLNYILLNLAVA D LFMV-FGGF T TTMYTSMH-- GYFVFGP TG C P.59.2 RHO1_takRu V N FLTLFVTVKHKKLRTPLNYVLLNLAVA D LFMV-IGGF T VTLYTALH-- AYFVLGV TG C P.59.2 RHO1_leuEr V N FLTLFVTIQHKKLRQPLNYILLNLAVS D LFMV-FGGF T TTIITSMN-- GYFIFGP AG C P.59.2 RHO1_calMi V N FLTLYVTFEHKKLRQPLNFILLNLAVA D LFMV-FGGF F ITVYTSLH-- GYFVFGV TG C P.59.2 RHO1_petMa V N FLTLFVTVQHKKLRTPLNYILLNLAVA N LFMV-LFGF T LTMYSSMN-- GYFVFGP TM C P.59.2 RHO1_letJa V N FLTLFVTVQHKKLRTPLNYILLNLAMA N LFMV-LFGF T VTMYTSMN-- GYFVFGP TM C P.59.2 RHO1_geoAu V N FLTLFVTVQHKKLRTPLNYILLNLAVS N LFMI-LFGF T TTMYTSMN-- GYFVFGP TM C P.59.2 RHO2_calMi I N GLTLLVTVKHKKLRQPLNFILLNLAVA D LFMV-FGGF F ITVYTSLH-- GYFVFGV TG C P.59.2 RHO2_galGa I N LLTLLVTFKHKKLRQPLNYILVNLAVA D LFMA-CFGF T VTFYTAWN-- GYFVFGP VG C P.59.2 RHO2_taeGu I N FLTLLVTFKHKKLRQPLNYILVNLAVA D LCMA-CFGF T VTFYTAWN-- GYFVFGP IG C P.59.2 RHO2_podSi I N LLTLLVTFKHKKLRQPLNYILVNLAVA D LFMA-CFGF T VTFYTAWN-- GYFIFGP IG C P.59.2 RHO2_anoCa I N ILTLLVTFKHKKLRQPLNYILVNLAVA D LFMA-CFGF T VTFYTAWN-- GYFIFGP IG C P.59.2 RHO2_neoFo I N LLTLVVTFKHKKLRQPLNYILVNLAVA D LFMV-CFGF T VTFSTAIN-- GYFIFGP RG C P.59.2 RHO2_latCh I N FLTLLVTFKHKKLRQPLNYILVNLAVA S LFMV-VFGF T VTFYSSLN-- GYFVLGP MG C P.59.2 RHO2_gekGe L N GLTLFVTFQHKKLRQPLNYILVNLAAA N LVTV-CCGF T VTFYASWY-- AYFVFGP IG C P.59.2 RHO2_pheMa L N GLTLFVTFQHKKLRQPLNYILVNLAVA N LLMV-ICGF T VTFYTSWY-- GYFVFGP MG C P.59.2 RHO2_geoAu V N FMTLFVTFKLKKLRQPLNFILVNLCVA D LLMI-MFGF T TTFYTAMN-- GYFVFGP TG C P.59.2 RHO2_danRe I N GLTLLVTAQHKKLRQPLNFILVNLAVA G TIMV-CFGF T VTFYSAIN-- GYFVLGP TG C P.59.2 RHO2d_danR I N GLTLLVTAQHKKLRQPLNFILVNLAVA G TIMV-CFGF T VTFYTAIN-- GYFVLGP TG C P.59.2 RHO2c_danR I N GLTLVVTAQHKKLRQPLNFILVNLAVA G TIMV-CFGF T VTFYTAIN-- GYFVLGP TG C P.59.2 RHO2a_danR I N VLTLVVTAQHKKLRQPLNYILVNLAFA G TIMV-IFGF T VSFYCSLV-- GYMALGP LG C P.59.2 RHO2b_danR I N VLTLLVTAQHKKLRQPLNYILVNLAFA G TIMA-FFGF T VTFYCSIN-- GYMALGP TG C P.59.2 RHO2_takRu I N GLTLLVTAQNKKLRQPLNYILVNLAVA G LIMC-AFGF T ITITSAVN-- GYFILGA TA C P.59.2 RHO2_gasAc I N GLTLLVTAQNKKLRQPLNYILVNLAVA G LIMC-AFGF T ITITSAVN-- GYFILGA TA C P.59.2 RHO2_oreNi I N GLTLFVTAQNKKLRQPLNYILVNLAVA G LIMC-CFGF T ITITSAIN-- GYFVLGT TF C P.59.2 RHO2_hipHi I N GLTLFVTAQNKKLRQPLNYILVNLAVA G LIMC-CFGF T ITITSAFN-- GYFILGA TF C P.59.2 RHO2_mulSu I N GLTLLVTFQNKKLQQPLNYILVNLAVV G LIMC-AFGF T ITITSALN-- GYFILGP TF C P.59.2 RHO2_pomMi I N ALTLLVTFQNKKLRQPLNFILVNLAVA G LIMC-AFGF T ITITSALN-- GYFILGA TF C P.59.2 RHO2_oryLa I N ALTLVVTAQNKKLRQPLNFILVNLAVA G LIMV-CFGF T VCIYSCMV-- GYFSLGP LG C P.59.2 SWS2_ornAn I N LLTVICTIKYKKLRSHLNYILVNLAVS N MLVV-CVGS A TAFYSFAH-- MYFVLGP TA C P.59.2 SWS2_anoCa I N VLTIFCTFKYKKLRSHLNYILVNLSVS N LLVV-CVGS T TAFYSFSN-- MYFSLGP TA C P.59.2 SWS2_utaSt I N VLTIFCTFKYKKLRSHLNYILVNLAVS N LLVV-CIGS T TAFYSFAQ-- MYFSLGP TA C P.59.2 SWS2_taeGu I N ALTVLCTAKYKKLRSHLNYILVNLAVA N LLVV-CVGS T TAFYSFSQ-- MYFALGP LA C P.59.2 SWS2_neoFo I N VLTIICTFKYKKLRSHLNYILVNLAVA N LIVV-GFGS T TAFYSFSQ-- MYFAWGP LA C P.59.2 SWS2_galGa I N TLTIFCTARFRKLRSHLNYILVNLALA N LLVI-LVGS T TACYSFSQ-- MYFALGP TA C P.59.2 SWS2_xenTr L N LLTIICTVKYKKLRSHLNYILVNLAVA N LIVI-CFGS T TAFYSFSQ-- MYFSLGT LA C P.59.2 SWS2_geoAu L N FLTVFVTIKYKKLRSHLNYILVNLAIA N LIVV-CCGS T LAFYSFMH-- KYFILGP LF C P.59.2 SWS2_takRu I N VLTIACTIQYKKLRSHLNYILVNLAFS N LLVT-TVGS F TCFCCFFV-- RYMIVGP LG C P.59.2 SWS2_gasAc I N ALTVACTVQNKKLRSHLNYILVNLAVS N LLVS-GVGA F TAFLSFAA-- RYFVLGT LA C P.59.2 SWS1_homSa L N AMVLVATLRYKKLRQPLNYILVNVSFG G FLLC-IFSV F PVFVASCN-- GYFVFGR HV C P.59.2 SWS1_ailMel L N ATVLVATLRYRKLRQPLNYILVNVSLA G FVYC-I-SV S TVFIASCH-- GYFIFGR HV C P.59.2 Caniformia-restricted deletion SWS1_canFam L N GTVLVATLRYKKLRQPLNYILVNVSLG G FLYC-I-SV S TVFIASCQ-- GYFVFGR HV C P.59.2 Caniformia-restricted deletion SWS1_felCat L N ATVLVATLRYRKLRQPLNYILVNVSLG G FLYC-VSSV S IVFITSCH-- AYFIFGR H VC P.59.2 SWS1_monDo L N AVVLVATLRYKKLRQPLNYILVNVSLC G FIFC-IFAV F TVFISSSQ-- GYFIFGR HV C P.59.2 SWS1_smiCr L N GVVLIATLRYKKLRQPLNYILVNISLA G FIFC-VFSV F TVFVSSSQ-- GYFVFGR HV C P.59.2 SWS1_tarRo L N AVVLIATLRYKKLRQPLNYILVNISLA G FIFC-VISV F TVFISSSQ-- GYFIFGR HV C P.59.2 SWS1_taeGu L N AIVLIVTIKYKKLRQPLNYILVNISVS G LMCC-VFCI F TVFIASSQ-- GYFVFGK HM C P.59.2 SWS1_anoCa L N AIILIVTVKYKKLRQPLNYILVNISFA G FLFC-TFSV F TVFMASSQ-- GYFFFGR HV C P.59.2 SWS1_utaSt L N AIILIVTVKYKKLRQPLNYILVNISFA G FLFC-VFSV F TVFLASSQ-- GYFFFGR HI C P.59.2 SWS1_neoFo L N AIVLFVTIKYKKLQQPLNYILVNISLA G FIFC-FFGV F AVFIASCQ-- GYFIFGK TV C P.59.2 SWS1_galGa L N AVVLWVTVRYKRLRQPLNYILVNISAS G FVSC-VLSV F VVFVASAR-- GYFVFGK RV C P.59.2 SWS1_xenLa L N FIVLLVTIKYKKLRQPLNYILVNITVG G FLMC-IFSI F PVFVSSSQ-- GYFFFGR IA C P.59.2 SWS1_petMa L N AIVLIVTVKCKKLRQPLTYMLVNISAA G LVFC-LFSI S TVFLFSTQ-- GYFVFGP TV C P.59.2 SWS1_geoAu L N AIVLVVTIKYKKLRQPLNYILVNISAA G LVFC-LFSI S TVFVASMQ-- GYFFLGP TI C P.59.2 SWS1_danRe M N GIVLFVTMKYKKLRQPLNYILVNISLA G FIFD-TFSV S QVSVCAAR-- GYYSLGY TL C P.59.2 SWS1_oryLa L N FVVLLATAKYKKLRVPLNYILVNITFA G FIFV-TFSV S QVFLASVR-- GYYFFGQ TL C P.59.2 LWS_homSap T N GLVLAATMKFKKLRHPLNWILVNLAVA D LAET-VIAS T ISVVNQVY-- GYFVLGH PM C P.59.2 LWS_ailMel T N GLVLAATMRFKKLRHPLNWILVNLAVA D LAET-VIAS T ISVVNQIY-- GYFVLGH PL C P.59.2 LWS_monDom T N GLVLVATMKFKKLRHPLNWILVNLAVA D LGET-VIAS T ISVINQIY-- GYFILGH PL C P.59.2 LWS_macEug T N GLVLVATMKFKKLRHPLNWILVNLAVA D LGET-LIAS T ISVINQIY-- GYFILGH PM C P.59.2 LWS_smiCra T N GLVLVATMKFKKLRHPLNWILVNLAVA D LGET-IIAS T ISVINQIY-- GYFILGH PM C P.59.2 LWS_ornAna T N GLVLVATMKFKKLRHPLNWILVNLAVA D LGET-LIAS T ISVINQIF-- GYFILGH PM C P.59.2 LWS_galGal T N GLVLVATWKFKKLRHPLNWILVNLAVA D LGET-VIAS T ISVINQIS-- GYFILGH PM C P.59.2 LWS_anoCar T N GLVLVATAKFKKLRHPLNWILVNLAIA D LGET-VIAS T ISVINQIS-- GYFILGH PM C P.59.2 LWS_xenTro T N GLVLVATLKFKKLRHPLNWILVNMAIA D LGET-VIAS T ISVCNQIF-- GYFVLGH PM C P.59.2 LWS_petMar S N GLVLVATVKFKKLRHPLNWIIVNLAIA D ILET-IFAS T ISVCNQVY-- GYFILGH PM C P.59.2 LWS_letJap T N GLVLVATMKFKKLRHPLNWILVNLAIA D ILET-IFAS T ISVCNQVF-- GYFILGH PM C P.59.2 LWS_geoAus T N GLVLVATLKFKKLRHPLNWILVNLAIA D IGET-IFAS T VSVVNQIF-- GYFILGH PL C P.59.2 LWS_neoFor T N GLVLMATYKFKKLRHPLNWILVNLAIA D LGET-LIAS T ISVTNQIF-- GYFILGH PM C P.59.2 LWS_takRub T N GLVLVATAKFKKLRHPLNWILVNLAIA D LGET-VFAS T ISVCNQFF-- GYFILGH PM C P.59.2 LWS_gasAcu T N GLVLVATAKFKKLQHPLNWILVNLAIA D LGET-VFAS T ISVCNQFF-- GYFILGH PM C P.59.2 LWS1_calMi T N GLVLVATVRFKKLRHPLNWILVNMALA D LGET-VLAS T VSVANQFF-- GYFILGH PL C P.59.2 LWS2_calMi T N GLVLVATWKFKKLRHPLNWILVNLAIA D LGET-LFAS T ISICNQVF-- GYFILGH PM C P.59.2 PIN_galGal V N GLVIVVSICYKKLRSPLNYILVNLAVA D LLVT-LCGS S VSLSNNIN-- GFFVFGR RM C P.59.2 PIN_colLiv V N GLVIVVSIRYKKLRSPLNYILVNLAMA D LLVT-LCGS S VSFSNNIN-- GFFVFGK RL C P.59.2 PIN_taeGut L N GLVIVVSVRHKRLRSPLNYILLNLAVA N LLVT-LCGS S VSLSNNIS-- GFFVFGE RL C P.59.2 PIN_utaSta V N GLVIVVSIQYKKLRSPLNYILVNLAIA D LLVT-SFGS T LSFANNIY-- GFFVLGQ TA C P.59.2 PIN_podSic V N GLVIVVSVQFKKLRSPLNYVLVNLAVA D LLVT-FFGS T ISFVNNAQ-- GFFIFGQ AT C P.59.2 PIN_pheMad A N GLVIAVSVRFKRLRSPLNYILVNLATA D LLVT-FFGS I ISFVNNAV-- GFFVFGK TA C P.59.2 PIN_xenTro V N GLVIVVTLKYKKLRSPLNYILVNLAIA N LLVT-IFGS S VSFSNNVV-- GYFFMGK TM C P.59.2 PIN_bufJap V N GMVIVVSLKYKKLRSPLNYILVNLAVA D ILVT-MFGS T VSFHNNIF-- GFFTLGK LV C P.59.2 VAOP_galGa E N LAVILVTFKFKQLRQPVNYVIVNLSVA D FLVS-LTGG T ISFLANLK-- GYFYMGH WA C P.59.2 VAOP_taeGu E N LAVILVTFKFKQLRQPINYIIVNLSVA D FLVS-LTGG T ISFLTNLK-- GYFFMGY WA C P.59.2 VAOP_anoCa E N FTVILVTIKFKQLRQPLNYVIVNLSVA D FLVS-LIGG T ISFSTNLK-- GYFYMGH WA C P.59.3 VAOP_xenTr E N FIVILVTAKFKQLRQPLNYIIVNLSVA D FLVS-VIGG T ISIATNSR-- GYFYLGS WA C P.59.2 VAOP_danRe E N FTVMLVTFRFQQLRQPLNYIIVNLSLA D FLVS-LTGG S ISFLTNYH-- GYFFLGK WA C P.59.2 VAOP_rutRu E N FAVMLVTFRFTQLRKPLNYIIVNLSLA D FLVS-LTGG T ISFLTNYH-- GYFFLGK WA C P.59.2 VAOP_takRu E N FLVMFITFKFKQLRQPLNYIIVNLAIA D FLVS-LTGG L ISFLTNAR-- GYFFLGR WA C P.59.2 VAOP_petMa E N FAVIVVTARFRQLRQPLNYVLVNLAAA D LLVS-AIGG S VSFFTNIK-- GYFFLGV HA C P.59.2 PPIN_anoCa L N TAVIAITIKYRQLRQPINYSLVNLAIA D LGAA-LLGG S LNVETNAV-- GYYNLGR VG C P.59.2 PPIN_xenTr L N VTVIVVTFKYRQLRHPINYSLVNLAIA D LGVT-VLGG A LTVETNAV-- GYFNLGR VG C P.59.2 PPINa_petM L N STVIIVTLRHRQLRHPLNFSLVNLAVA D LGVT-VFGA S LVVETNAV-- GYFNLGR VG C P.59.2 PPIN_letJa L N STVVIVTLRHRQLRHPLNFSLVNLAVA D LGVT-VFGA S LVVETNAV-- GYFNLGR VG C P.59.2 PPIN_danRe L N VTVITVTLKYKQLRQPLNFALVNLAVA D LGCA-VFGG L PTVVTNAM-- GYFSLGR VG C P.59.2 PPIN_ictPu L N MVVIIVTVRYKQLRQPLNYALVNLAVA D LGCP-VFGG L LTAVTNAM-- GYFSLGR VG C P.59.2 PPIN_oncMy M N VLVIMVTMRHRKLRQPLNYALVNLAVA D LGCA-LFGG L PTMVTNAM-- GYFSMGR LG C P.59.2 PPINb_takR L N VLVIVVTMKHRQLRQPLSYALVNLAIC D LGCA-LFGG I PTTITSAM-- GYFSLGR VG C P.59.2 PPINb_tetN L N VLVIVVTLKHRQLRQPLNYALVNLAIC D LGCA-LFGG I PTTVTSAM-- GYFSLGR LG C P.59.2 PPINb_gasA L N ALVIVVTARHRQLRQPLSYALVNLAVC D LGCA-ACGG L PTTVTSAM-- GYFSLGR AG C P.59.2 PPINa_gasA L N ATVIIVTLMHKQLRQPLNYALVNMALA D LGTA-MTGG V LSVVNNAQ-- GYFSLGR SG C P.59.2 PPINa_takR L N ATVIIVSLMHKQLRQPLNYALVNMAVA D LGTA-MTGG L LSVVNNAQ-- GYFSLGR TG C P.59.2 PPINa_tetN L N ATVIIVSLMHKQLRQPLNYALVNMAAA D LGTA-VSGG L LSVVNNAQ-- GHFSLGR TG C P.59.2 PPINa_cioI L N ILVIVATLKNKVLRQPLNYIIVNLAVV D LLSG-FVGG F ISIAANGA-- GYFFWGK TM C P.59.2 PPINa_cioS L N ILVITATLKNKVLRQPLNYIIVNLAVV D LLSG-LVGG V ISIFANGA-- GYFFWGK FM C P.59.2 PPINb_cioI L N GFVIIATMKNKKLRQPLNYIIINLSIA D FLSG-LVGG F IGMISNSA-- GYFYFGK TV C P.59.2 PPINb_cioS L N LLVIVATYKNKDLRRPINYIIVNLAVA D LTCS-VVGG L LGVLNNGA-- GYYFLGK SV C P.59.2 PARIE_utaS N N SLVIAVTLKNPQLRNPINIFILNLSFS D LMMS-LCGT T IVIATNYY-- GYFYLGR KF C P.59.2 PARIE_anoC N N FLVIAVTLKNPQLRNPINIFILNLSFS D LMMS-ICGT T IVIATNYH-- GYFYLGR RF C P.59.2 PARIE_xenT N N AIVILVTLKHPQLRNPINIFILNLSFS D LMMA-LCGT T IVVSTNYH-- GYFYLGK QF C P.59.2 PARIE_takR N N SLAIAVMLKNPSLLQPINIFILSLAVS D LMIG-LCGS L VVTITNYH-- GSFFIGH TA C P.59.2 PARIE_tetN N N GLAITVMLKNPALLQPINIFILSLAVS D LMIG-LCGS L VVTITNYQ-- GSFFIGH TA C P.59.2 PARIE_gasA N N VLVITVLVRNPSLLQPMNVFILSLAVS D LMIG-LCGS L VVTITNYH-- GSFFIGH TA C P.59.2 PARIE_danR N N VLVIAVMVKNLHFLNAMTVIIFSLAVS D LLIA-TCGS A IVTVTNYE-- GSFFLGD AF C P.59.2 ENCEPH_hom N N LLVLVLYYKFQRLRTPTHLLLVNISLS D LLVS-LFGV T FTFVSCLR-- NGWVWDT VG C P.59.2 ENCEPH_oto N N LLVLVLYYKFPRLRTPTHLFLVNISLS D LLVS-LFGV T FTFVSCLR-- NGWVWDT VG C P.59.2 ENCEPH_lox N N LLVLVLYYKFQRLRTPTHLFLVNISLS D LLVS-LFGV T FTFVSCLR-- NGWVWDT VG C P.59.2 ENCEPH_pte N N LLVLVFYYKFQQVRTPFYLFLVNISFS D LLVS-FFGV T FTFVSCLR-- NGWVWDT VG C P.59.2 ENCEPH_mus G N LLVLLLYSKFPRLRTPTHLFLVNLSLG D LLVS-LFGV T FTFASCLR-- NGWVWDA VG C P.59.2 ENCEPH_can C H FCPQKGFLEFQRLRTPTHLLLVNLSLS D LLVS-LFGV T FTFVSCLR-- NGWVWDS VG C P.59.2 ENCEPH_mon N N LLVLVLYYKFQRLRTPTHLFLVNISFN D LLVS-LFGV T FTFVSCLR-- SGWVWDS VG C P.59.2 ENCEPH_ano N N LLVLVLYAKFKRLRTPTHLFLVNISLS D LLVS-LFGV S FTFGSCLR-- HRWVWDA AG C P.59.2 ENCEPH_gal N N LLVLVLYYKFKRLRTPTNLFLVNISLS D LLVS-VCGV S LTFMSCLR-- SRWVWDA AG C P.59.2 ENCEPH_dan N N IIVIILYSRYKRLRTPTNLLIVNISVS D LLVS-LTGV N FTFVSCVK-- RRWVFNS AT C P.59.2 ENCEPH_tak N N FVVLALYCRFKRLRTPTNLLLVNISLS D LLVS-LFGI N FTFAACVQ-- GRWTWTQ AT C P.59.2 ENCEPH_gas N N VVVIVLYCKFKRLRTPTNLLVVNISLS D LLVS-VIGI N FTFVSCIR-- GGWTWSR AT C P.59.2 ENCEPH_ory N N LLVILLYCKFKRLRTPTSLLLVNISLS D LLVS-VVGI N FTLASCVK-- GRWMWSQ AT C P.59.2 ENCEPH_xen N N LLVLILYCKFKRLQTPTNLLFFNTSLC H FVFS-LLAI T FTFMSCVR-- GSWAFSV EM C P.59.2 ENCEPH_cal N N ILVLLLYYKFKRLRTPTNLLLVNISVS D LLVS-VFGL S FTFVSCTQ-- GRWGWDS AA C P.59.2 ENCEPH_squ N N LLMLVLYCKFKRLRTPTNLFLVNISIS D LLLS-VFGV I FTFVSCVK-- GRWVWDS AA C P.59.2 ENCEPH_pet N N LLLVALFVGFKRLQTPTNLLLVNISLS D LLVS-VFGN T LTLVSCVR-- RRWVWGN GG C P.59.2 ENCEPH4_br N N FVVILLIGCHRQLRTPFNLLLLNMSVA D LLVS-VCGN T LSFASAVR-- HRWLWGR PG C P.59.2 ENCEPH4_br N N FVVILLIGCHRQLRTPFNLLLLNVSVA D LLVS-VCGN T LSFASAVQ-- HRWLWGR PG C P.59.2 TMT5_braFl S N GAVVLLFLKFRQLRTPFNMLLLNMSVA D LLVS-VCGN T LSFASAVR-- HRWLWGR PG C P.59.2 TMT5_braBe S N GAVVVLFLKFPQLRTPFNLLLLNMAVA D LLVS-VCGN T LSFASAVR-- HRWLWGR PG C P.59.2 TMT_monDom S N FIVLVLFCKFKVLRNPVNMLLLNISIS D MLVC-LSGT T LSFASSIQ-- GRWIGGK HG C P.59.2 TMT_macEug N N FIVLVLFCKFKVLRNPVNMLLLNISIS D MLVC-LTGT T LSFASSIR-- GRWIAGY HG C P.59.2 TMT_galGal N N LIVLILFCKFKTLRNPVNMLLLNISIS D MLVC-ISGT T LSFASNIH-- GKWIGGE HG C P.59.2 TMT_taeGut N N LIVLILFCKFKTLRNPVNMLLLNISVS D MLVC-ISGT T LSFASNIR-- GKWIGGD HA C P.59.2 TMT_anoCar N N LVVLILFCKFKTLRNPVNMLLLNISAS D MLVC-ISGT T LSFVSNIY-- GRWIGGE HG C P.59.2 TMT_xenTro N N FVVLILFCKFKTLRTPVNMMLLNISAS D MLVC-VSGT T LSFTSSIK-- GKWIGGE YG C P.59.2 TMT_ornAna N N LIVLILFCKFKALRNPVNMIMLNISAS D MLVC-VSGT T LSFASNIS-- GRWIGGD PG C P.59.2 TMT_danRer N N LVVLVLFCKFKTLRTPVNMLLLNISIS D MLVC-MFGT T LSFASSVR-- GRWLLGR HG C P.59.2 TMT_tetNig N N FIVLLLFCKFKKLRTPVNVLLLNISVS D MLVC-LFGT T LSFASSLR-- GRWLLGR SG C P.59.2 TMT_takRub N N FVVLLLFCKFKKLRTPVNMLLLNISVS D MLVC-LFGT T LSFASSIR-- GRWLLGR IG C P.59.2 TMT_gasAcu N N LVVLLLFCKFKKLRTPVNMLLLNISVS D MLVC-LFGT T LSFASSLR-- GKWLLGR SG C P.59.2 TMT_oryLat N N FVVLILFCKFKKLRTPVNMLLLNISVS D MLVC-LFGT T LSFASSIR-- GRWLLGR GG C P.59.2 TMTa1_anoC N N LLVLVLFCRNKVLRSPINLLLMNISLS D LMIC-IVGT P FSFAASTQ-- GKWLIGP AG C P.59.2 TMTa1_xenT N N LVVLILFCQYKVLRSPINMLLMNISLS D LMVC-ILGT P FSFAASTQ-- GHWLIGE IG C P.59.2 TMTa1_danR N N LLVLVLFGRYKVLRSPINFLLVNICLS D LLVC-VLGT P FSFAASTQ-- GRWLIGD TG C P.59.2 TMTb_danRe N N TLVLVLFCRYKVLRSPMNCLLISISVS D LLVC-VLGT P FSFAASTQ-- GRWLIGR AG C P.59.2 TMTa_gasAc N N LLVLVLFCRYKMLRSPINLLLINISIS D LLVC-VLGT P FSFAASTQ-- GRWLIGE GG C P.59.2 TMTb_gasAc S N FLVLALFCRYRALRTPMNLLLVSISAS D LLVS-MVGT P FSFAASTQ-- GRWLIGR AG C P.59.2 TMTa_oryLa N N LLVLVLFCRYKILRSPINLLLINISIS D LLVC-VLGT P FSFAASTQ-- GRWLIGE GG C P.59.2 TMTb_oryLa S N LLVLALFCRYRALRTPMNLLLVSISVS D LLVS-VLGT P FSFAASTQ-- GRWLIGR AG C P.59.2 TMTa_pimPr N N TLVLILFCRYKVLRSPMNYLLVSIAVS D LLVC-VLGT P FSFAASTQ-- GRWLIGR AG C P.59.2 TMTa_takRu N N LLVLVLFCRYKMLRSPINLLLMNISIS D LLVC-VLGT P FSFAASTQ-- GRWLIGE AG C P.59.2 TMTb_takRu S N FLVLALFCRYRALRTPMNLMLVSISAS D LLVS-VLGT P FSFAASTQ-- GRWLIGR AG C P.59.2 TMTa_tetNi S N LLVLVLFCRFKVLRSPINLLLVNISVS D LLVC-VLGT P FSFAASTQ-- GRWLIGA AG C P.59.2 TMTb_tetNi S N LLVLALFCRFRALRTPMNLMLVSISAS D LLVS-VLGT P FSFAASTQ-- GRWLLGR AG C P.59.2 TMTa_oncMy S N LFVLLVFARFQVLRTPINLILLNISVS D MLVC-IFGT P FSFAASLY-- GRWLIGA HG C P.59.2 TMTa1_calM N N LLVLVLFCKYKVLRSPMNMLLLNISVS D MLVC-ICGT P FSFAASVQ-- GRWLVGE QG C P.59.2 TMTa2_calM N N LLVLLLFVCFKEIRTPLNMILLNISLS D LSVC-VFGT P FSFAASIY-- RRWLIGH KG C P.59.2 TMTx_braFl N N STTLYLVGRYKQLRTPFNILMVNLSVS D LLMC-VLGT P FSFVSSLH-- GRWMFGH SG C P.59.2 TMTPIN_str N N GIVMILFARFPSLRHPINSFLFNVSLS D LIIS-CLAS P FTFASNFA-- GRWLFGD LG C P.59.2 TMTy_braFl T N LLTVLVFWCFKSLRTPFHLYLGGIALS D LLVA-ALGS P FAVASAVG-- ERWLFGR AV C P.59.2 ENCEPH_str G N SVVLFLFAWDRHLRTPTNMFLLSLTIS D WLVT-VVGI P FVTASIYA-- HRWLFAH VG C P.59.2 TMT_apiMel A N LLVAIVIVKDAQLWTPVNVILFNLVFG D FLVS-IFGN P VAMVSAAT-- GGWYWGY KM C P.59.2 TMT1_anoGa L N IFVIALMYKDVQLWTPMNIILFNLVCS D FSVS-IIGN P LTLTSAIS-- HRWLYGK SI C P.59.2 TMT2_anoGa L N LFVIALMCKDMQLWTPMNIILFNLVCS D FSVS-IIGN P LTLTSAIS-- HRWIFGR TL C P.59.2 TMT_aedAeg L N LFVIALMCKDVQLWTPINIILFNLVCS D FSVS-IIGN P FTLTSAIS-- RHWIFGR TV C P.59.2 TMT_culPip L N LFVIALMCKEVQLWTPMNIILLNLVCS D FSVS-IVGN P FTLSSAIS-- HRWLFGR KL C P.59.2 TMT_triCas L N LTVIIFMLKERQLWSPLNIILFNLVVS D FLVS-VLGN P WTFFSAIN-- YGWIFGE TG C P.59.2 TMT_bomMor L N LMVILLMFKDRQLWTPLNIILFNLVCS D FSVS-VLGN P FTLISALF-- HRWIFGH TM C P.59.2 TMT_helVir L N LMVILLMFKDRQLWTPLNIILFNLVCS D FSVS-VLGN P FTLISALF-- HRWIFGK TM C P.59.2 TMT_rhoPro G N LIVIIIMCRDKNLWTPVNFILFNVIVS D FSVA-ALGN P FTLASAIA-- KRWFFGQ SM C P.59.2 TMT_acyPis F N TCVIFIMIRDTRLWTPQNVIIFNLATS D LAVS-VLGN P VTLAAAIT-- KGWIFGQ TI C P.59.2 TMTa_dapPu M N IVVVVIILNDSQKMTPLNWMLLNLACS D GAIA-GFGT P ISAAAALK-- FTWPFSH EL C P.59.2 TMTb_dapPu M N VVVVIVILNDSQRMTPLNWMLLNLACS D GAIA-GFGT P ISTAAALE-- FGWPFSQ EL C P.59.2 TMT1_plaDu S N GVIMYLYFKDKSLRSPMNLLFVNLAMS D FTVA-FFGA M FQFGLTCTR- KYMSPGM AL C P.59.1 TMT2_plaDu L N VLVLVLFIKDRKLRSPNNFLYVSLALG D LLVA-VFGT A FKFIITARK- TLLREED GF C P.59.1 TMT_triCys L N GLVIAVLIKYIRTITNTNIIVLSMSCA N ILIP-LLGS P LSATSSLM-- RKWQFGN GG C P.59.2 CUBOP_carR L N MIVLITFYRLRHKLAFKDALMASMAFS D VVQA-IVGY P LEVFTVVD-- GKWTFGM EL C P.59.2 MEL1_acrMi L N SVVILTFLLDRSLLFPANLIILSIAIS D WLMS-VVPN I MGGVANAS-- NDLPFTD WS C P.59.2 ENC_nemVec T N TIVVIIFISSQRLHTTPNLILFSMSVC D WLMA-TMAK S VGIYGNAR-- YWPTVGK VT C P.59.2 ENC_nemVec T N TIVVITFIFSKRLHTTPNLILFSMSVC D WLMA-AMAK S VGIYGNAR-- YWPTVGK VT C P.59.2 ENC_nemVec L N GIVLIIFLATRSLRTIPNMILLSMAWA D WLMA-CLAD A VGAYANAN-- NWPSMVG GL C P.59.2 RGR1_homSa L N TLTIFSFCKTPELRTPCHLLVLSLALA D SGIS-LNAL V AATSSLL--- RRWPYGS DG C P.59.3 RGR1_ornAn L N GLTIASFRKIKELRTPSNLLVVSLALA D SGIC-LNAL M AALSSFL--- RHWPYGA EG C P.59.3 RGR1_galGa L N GLTIISFRKIKELRTPSNLLVLSIALA D CGIC-INAF I AAFSSFL--- RYWPYGS EG C P.59.3 RGR1_xenTr L N GLTLLSFYKIRELRTPSNLFIISLAVA D TGLC-LNAF V AAFSSFL--- RYWPYGS EG C P.59.3 RGR1_gasAc L N AVTIAAFLKVRELRTPSNFLVFSLAVA D IGIS-MNAT I AAFSSFL--- RYWPYGS DG C P.59.3 RGR2_danRe L N AISVLAFLRVREMQTPNNFFIFNLAVA D LSLN-INGL V AAYACYL--- RHWPFGS EG C P.59.3 RGR2_pimPr L N LISVLAFLRVREIQTPNNFFIFNLAVA D LSLN-INGL V AAYASYL--- RYWPFGS EG C P.59.3 RGR2_tetNi L N AISIVSFLTVKEMRNPSNFFVFNLALA D ISLN-VNGL I AAYASYL--- RYWPFGQ DG C P.59.3 RGR2_gasAc L N AISIASFLRVKEMWNPSNFFVFNLAVA D ICLN-VNGL T AAYASYL--- RYWPFGQ DG C P.59.3 RGR2_oryLa L N AISILAFLRVKEMRSPSSFLVFNLALA D ISLN-INGL T AAYASYL--- RYWPFGQ EG C P.59.3 RGR1_calMi L N GLTLLAFYKIKELRTPSNLLITSLALS D FGIS-MNAF I AAFSSFL--- RYWPYGS EG C P.59.3 RGRa_cioIn G Y SLLFVIFAKRPDLKK-KNKFLLSLATS D LLIT-VHVF A STIAAFA--- PQWPFGD LG C P.59.3 RGRa_cioSa G Y GLLFVIFAKSPDLKK-KNRFLFSLAVS D LLIT-IHVV A SVVASFQ--- SEWPFGS IG C P.59.3 RGRb1_cioI G Y AVYFGAIWRSKTLQT-RHIWLTSLACG D IIMM-VHLI L ESLSSLGM-- GHRPRQN FE C P.59.2 RGRb2_cioI G Y SVYILAIWSSKKLQT-KHIWLTSLACA D LLMM-VHLF M DGLSSFHQ-- GRRPKGI FE C P.59.2 RGRb2_cioS G Y SIYLRAIWSSRKLQT-RHIWLTSLACA D LIMM-VHLF M DGLSSFHQ-- GRRPKGN FE C P.59.2 PER1_lotGi L S LLVALTFIREKGLFKYGRAWLHISLAI A NVGV-VGAF P FSGSSSFS-- GRWLYGS GM C P.59.2 PER1_aplCa L N LLTALTFYKDTKLTKGSQPWLHILLAL A NVGV-VAPS P FPASSSFS-- GRWLYGS TM C P.59.2 PER1_todPa L C GMCIIFLARQSPKPRRKYAILIHVLIT A MAV--NGGD P AHASSSIV-- GRWLYGS VG C P.58.2 PER1_homSa S N IIVLGIFIKYKELRTPTNAIIINLAVT D IGVS-SIGY P MSAASDLY-- GSWKFGY AG C P.59.2 PER1_ornAn S N VIVLGIFVKFEELRTATNAIIINLAVT D IGVS-GIGY P MSAASDLH-- GSWKFGH AG C P.59.2 PER1_monDo S N VIVLGIFVKYKALRTATNTIIINLAVT D IGVS-SIGY P MSAASDLY-- GSWKFGY DG C P.59.2 PER1_xenTr S N IIVLGIFVKYKELRTATNAIIINLAFT D IGVS-GIGY P MSAASDLH-- GSWKFGY VG C P.59.2 PER1_gasAc S N IVVLLMFWKFKELRTATNFIIINLAFT D IGVA-GIGY P MSAASDIH-- GSWKFGY AG C P.59.2 PER1a_sacK L S SVNFRMLLSNPDYCSKAGNFFLSLAVT D LCVC-IFET P FSAFSHHA-- GFWIFGD TA C P.59.2 PER1b_sacK G N SVVLEMFRRYKELLSPSAILLISLALA D LGLT-IFGM S LSCVSSFA-- GRWLFGK FG C P.59.2 PER3_braFl E N GITLATFTKFRSLRSPTTMLLVHLAIA D LGIC-IFGY P FSGASSLR-- SHWLFGG VG C P.59.2 PER3_braBe E N GITLATFSKFRSLRSPTTMLLVHLAIA D LGIC-IFGY P FSGASSLR-- SHWLFGG VG C P.59.2 PER3_hadAd G N GLVLVTFLRFRVLVTPTTLLLVNLAVS D LGLI-LFGF P FSASSSLS-- AKWIFGE GG C P.59.2 PER2_patYe G N LLIIIVFAKRRSVRRPINFFVLNLAVS D LIVA-LLGY P MTAASAFS-- NRWIFDN IG C P.59.2 PER1_braFl G N IFAIIVFLTEKEFRKKEHNSFALNLAIA D LSVCVFAY P SSTISGYA-- GEWMLGD VG C P.60.2 PER1_braBe G N VITITVFLTEKEFRKKQQNGFVLNLAIA D LSVCVFAY P SSAIAGYA-- GRWVLGD VG C P.60.2 PER2_braFl G N ATVVLMFMLKWRQLCRKANLLIINLAAV D LCISVFGY P FSASSGFA-- NQWLFSD AI C P.60.2 PER2_braBe G N ATVVLMFIMKWRQLCRKANLLVINLAAA N LCITIFGY P FSASSGYA-- HQWLFPD AI C P.60.2 PER2a_strP G N ITVICVLCRYRTFRKRSINLLLINMAAS D LGVSVAGY P LTTVSGYW-- GRWLFGD VG C P.60.2 PER2b_strP G N ITVLCVLCRYGTFRKRSVNILLMNMAVS D LGVSVAGY P LTAISGYR-- GRWVFAD IG C P.60.2 NEUR1_homS G N GYVLYMSSRRKKKLRPAEIMTINLAVC D LGIS-VVGK P FTIISCFC-- HRWVFGW IG C P.59.2 NEUR1_calJ G N GYVLYMSSRRKKKLRPAEIMTINLAVC D LGIS-VVGK P FTIISCFC-- HRWVFGW IG C P.59.2 NEUR1_dasN G N GYVLYMSSKRKKKLRPAEIMTINLAVC D LGIS-VVGK P FTIISCFC-- HRWVFGW IG C P.59.2 NEUR1_canF G N GYVLYMSSRRKKKLRPAEIMTINLAIC D LGIS-VVGK P FTIISCFC-- HRWVFGW IG C P.59.2 NEUR1_bosT G N GYVLYMSSRRKKKLRPAEIMTVNLAIC D LGIS-VVGK P FTIISCFC-- HRWVFGW IG C P.59.2 NEUR1_musM G N GYVLYMSSRRKKKLRPAEIMTINLAVC D LGIS-VVGK P FTIISCFC-- HRWVFGW FG C P.59.2 NEUR1_loxA G N GYVLYMSCRRKKKLRPAEIMTINLAVC D LGIS-VVGK P FVIISCFC-- HRWVFGW IG C P.59.2 NEUR1_ochP G N GYVLYMSSRRKKKLRPAEIMTINLAVC D LGIS-VVGK P FTIISCFC-- HRWVFGW IG C P.59.2 NEUR1_monD G N GYVIYMSSKRKKKLRPAEIMTVNLAVC D LGIS-VVGK P FTIISCFS-- HRWVFGW VG C P.59.2 NEUR1_ornA G N GYVIYMSSRRKKKLRPAEIMTVNLAVC D LGIS-VVGK P FTIVSCFC-- HRWVFGW MG C P.59.2 NEUR1_galG G N GYVIFMSSKRKKKLRPAEIMTVNLAVC D LGIS-VVGK P FSIISFFS-- HRWIFGW MG C P.59.2 NEUR1_xenT G N GYVIYMACSRKKKLRPAEIMTINLAVC D LGIS-VTGK P FAIVSCFS-- HRWVFGW NA C P.59.2 NEUR1_danR G N GYVMYMTFKRKTKLKPPEIMTLNLAIF D FGIS-VSGK P FFIVSSFS-- HRWLFGW QG C P.59.2 NEUR1_calM G N GYVIYLSITQKRKLKPPEILITNLAIS D FGMS-VGGQ P FLIISCFS-- HRWIFGW VG C P.59.2 NEUR1a_bra G N GRVLWLSYRCRARLRPVEMFVVSLAVA D VGLS-LVGH P FAAASSLM-- GRWSFGS AG C P.59.2 NEUR1b_bra G N GRVLWLSYRNWAKLRPVELFVVSLAVT D VGIS-VFGY P FAASSSLL-- GRWSFGS AG C P.59.2 NEUR_strPu G N ISVIVISLRKREKLKPIDLLTINLAIA D FLIC-VVSY P LPMISAFR-- HRWSFGK FG C P.59.2 NEUR2_galG G N SILLYISYKKKHLLKPAEYFIINLAIS D LAMT-LTLY P LAVTSSLS-- HRWLYGK HI C P.59.2 NEUR2_anoC G N SILLYVSYKKKNLLKPAEYFMINLAIS D LGMT-LTLY P LAVTSSLA-- HRWLFGQ QV C P.59.2 NEUR2_xenT G N SMLLLVAYRKRSILKPAEFFIVNLSIS D LGMT-GTLF P LAIPSLFA-- HRWLFDK VT C P.59.2 NEUR2_danR G N GMLLFVAYRKRSSLKPAEFFVVNLSVS D LGMT-LSLF P LAIPSALA-- HRWLFGE IT C P.59.2 NEUR2_calM G N SVLLFVAYRKRQILKPAEYFVANLAVS D ISMT-VTLL P LAISSNFS-- HRWLFVS KP C P.59.2 NEUR3_galG G N SAVLATAVKRSSLLKSPELLTVNLAVA D IGMA-ISMY P LAIASAWN-- HAWLGGD AS C P.59.2 NEUR3_taeG G N SAVLATAVKRSSLLKPPELLTVNLAVA D IGMA-LSMY P LAIASAWS-- HAWLGGD AS C P.59.2 NEUR3_xenT G N CAVLATAVKCSSHLKAPDLLSINLAVA D LGMA-ISMY P LAIASAWN-- HAWLGGD AS C P.59.2 NEUR3_anoC G N SMVLAVAVKRSSCLRSPELLTVNLAAT D LGMG-LSMY P LAIASAWN-- HAWLGGE AT C P.59.2 NEUR3b_dan G N LMVLVMAYKRSNHMKPPELLSVNLAVT D LGAA-VTMY P LAVASAWN-- HHWIGGD VS C P.59.2 NEUR3a_dan G N AAVLLTAAWRHSVLKAPELLTVNLAVT D IGMA-LSMY P LSIASAFN-- HAWIGGD PS C P.59.2 NEUR3a_tet G N ASVLFSASRRLTPLKAPELLTVNLAVT D IGMA-LSMY P LSIASAFN-- HAWMGGD TA C P.59.2 NEUR3_petM G N GAVLGVAARRWAKLKAPELLSVNLALT D LGIA-ASIY P LAVASAWN-- HRWLGGQ PV C P.59.2 NEUR4_ornA G N SMVIFILHRQRGILNPTDYLTFNLAVS D ASVS-VFGY S RGIIEIFNVF RDDGFSI WT C P.62.0 NEUR4_galG G N SVVIFVLYKQRHLLQPTDYLTFNLAVS D ASIS-VFGY S RGIIEIFNVF RDDGFSI WT C P.62.0 NEUR4_taeG G N SIVIFVLYKQRHVLQPTDYLTFNLAVS D ASIS-VFGY S RGIIEIFNVF RDDGFSI WT C P.62.0 NEUR4_anoc G N SIVIFVLYRQRAGLQPTDYLTFNLAVS D ASVS-VFGY S RGIIEIFNVF RDDGFSI WT C P.62.0 NEUR4_xenT G N SIVIFVLYKQRANLLPTDYLTFNLAVS D ASTS-VFGY S RGIIEIFNVF RDDGFSI WT C P.62.0 NEUR4_danR G N SIVIFVLFRQRSTLQPTDYLTLNLAVS D ASIS-VFGY S RGILEIFNIF KDSGYSV WT C P.62.0 NEUR4_tetN G N TVVLFVLVRQRSSLQPTDLLTFNLAVS D ASIS-VFGY S RGIIQIFNVF QDSGFSI WT C P.62.0 NEUR4_gasA G N SLVMFVLYRQRASLQSTDFLTLNLAIS D ASIS-IFGY S RGILEIFNIF NDDGTWI WT C P.62.0 NEUR4_calM G N SIVIFILYRQRLSLQPPDYLTLNLAVS D ASIS-IFGY S RGIIEIFNVF RDDGFSI WT C P.62.0 UROPS1_tri G N MLVFLTFYKHASLRTTSNLFIINLAIT D LLTG-GIKD T LFIYGLTS-- YNWPKSA IL C P.58.2 UROPS2_tri G N GAVLLVLRYHHDDIKSASNYFITNLALTD FLLG-VLCM P CILISCLN-- GQWVFGQ TL C P.58.2 GPR17_homS G N TLALWLFIRDHKSGTPANVFLMHLAVA D LSCV--LVL P TRLVYHFSG- NHWPFGE IA C P.58.1 CYSLTR1_ho G N GFVLYVLIKTYHKKSAFQVYMINLAVA D LLCV--CTL P LRVVYYVHK- GIWLFGD FL C P.58.1 P2RY8_homS G N LFSLWVLCRRMGPRSPSVIFMINLSVT D LMLA--SVL P FQIYYHCNR- HHWVFGV LL C P.58.1 BDKRB2_hom E N IFVLSVFCLHKSSCTVAEIYLGNLAAA D LILA--CGL P FWAITISNN- FDWLFGE TL C P.58.1 SSTR1_homS G N SMVIYVILRYAKMKTATNIYILNLAIA D ELLM--LSV P FLVTSTLL-- RHWPFGA LL C P.58.2 OPRL1_homS G N CLVMYVILRHTKMKTATNIYIFNLALA D TLVL--LTL P FQGTDILL-- GFWPFGN AL C P.58.2 OPRM1_homS G N FLVMYVIVRYTKMKTATNIYIFNLALA D ALAT--STL P FQSVNYLM-- GTWPFGT IL C P.58.2 CCR4_homSa G N SVVVLVLFKYKRLRSMTDVYLLNLAIS D LLFV--FSL P FWGYYAA--- DQWVFGL GL C P.58.3 TACR2_homS G N AIVIWIILAHRRMRTVTNYFIVNLALA D LCMA-AFNA A FNFVYASH-- NIWYFGR AF C P.59.2 GALR1_homS G N SLVITVLARSKKPRSTTNLFILNLSIA D LAYL-LFCI P FQATVYAL-- PTWVLGA FI C P.59.2 QRFPR_homS G N ALVFYVVTRSKAMRTVTNIFICSLALS D LLIT-FFCI P VTMLQNIS-- DNWLGGA FI C P.59.2 PPYR1_homS G N LCLMCVTVRQKEKANVTNLLIANLAFS D FLMC-LLCQ P LTAVYTIM-- DYWIFGE TL C P.59.2 NPY1R_homS G N LALIIIILKQKEMRNVTNILIVNLSFS D LLVA-IMCL P FTFVYTLM-- DHWVFGE AM C P.59.2 GPR19_homS G N SLVCLVIHRSRRTQSTTNYFVVSMACA D LLIS-VAST P FVLLQFTT-- GRWTLGS AT C P.59.2 HCRTR1_hom G N TLVCLAVWRNHHMRTVTNYFIVNLSLA D VLVT-AICL P ASLLVDIT-- ESWLFGH AL C P.59.2 GPR161_hom G N LVIVVTLYKKSYLLTLSNKFVFSLTLS N FLLS-VLVL P FVVTSSIR-- REWIFGV VW C P.59.2 ADRA1D_hom G N LLVILSVACNRHLQTVTNYFIVNLAVA D LLLS-ATVL P FSATMEVL-- GFWAFGR AF C P.59.2 ADRB2_homS G N VLVITAIAKFERLQTVTNYFITSLACA D LVMG-LAVV P FGAAHILM-- KMWTFGN FW C P.59.2 ADRB1_melG G N VLVIAAIGSTQRLQTLTNLFITSLACA D LVVG-LLVV P FGATLVVR-- GTWLWGS FL C P.59.2 PRLHR_homS G N CLLVLVIARVRRLHNVTNFLIGNLALS D VLMC-TACV P LTLAYAFEP- RGWVFGG GL C P.59.1 NMUR2_homS G N VLVCLVILQHQAMKTPTNYYLFSLAVS D LLVL-LLGM P LEVYEMWRN- YPFLFGP VG C P.59.1 ADORA2A_ho G N VLVCWAVWLNSNLQNVTNYFVVSLAAA D IAVG-VLAI P FAITIS---- TGFCAAC HG C P.59.4 TRHR_homSa G N IMVVLVVMRTKHMRTPTNCYLVSLAVA D LMVLVAAGL P NITDSIY--- GSWVYGY VG C P.60.3 NPY2R_homS G N SLVIHVVIKFKSMRTVTNFFIANLAVA D LLVN-TLCL P FTLTYTLM-- GEWKMGP VL C P.59.2
Indels in the TM4-EC2-TM5 region
The retinal plug loop region EC2 contains the second half of the extracellular disulfide bond which is very important to the overall structural stability of the GPCR molecule. After years of back and forth, it was eventually concluded that 92% of gene family members, including all opsin though not all GPCR do contain a disulfide linking extracellular domain EC2 to TM3.
That requires more than just comparative genomic conservation of the two cysteines because overall conservation can be quite high. Furthermore, it is quite difficult to establish homological correspondences in EC2 because of gapping issues (see alignment below). However non-existence or non-conservation of the cysteine does establish absence. All of the opsin outgroup GPCR considered here do contain a disulfide. The disulfide thus is not at all diagnostic for opsin-class GPCR and its absence reliably implies sequencing error.
These regions can only be aligned after careful determination of anchor residues on both sides of the disulfide cysteine. Some are subtle, involving small reduced alphabets of similar residues and imperfect invariance rather than absolute conservation. The anchors consist of a universally conserved W in TM4, a proline prior to TM4 exiting the membrane, the GWS.Y..E region unique to opsins, an aromatic residue 3 residues after the C, and finally the reliable P......CY region well within TM5.
This region has been quite permissive with regards to indels over evolutionary time. To account for observed length variations, six indels are needed within opsins and dozens more for the full spectrum of rhodopsin-class GPCR. Unsurprisingly almost all of these can be assigned to the EC2 extracellular loop rather than to TM4 or TM5. Somehow the disulfide bond has been able to accommodate these abrupt length variations without loss of steric capacity for formation, suggesting the loop is otherwise weakly constrained.
However more than the disulfide and membrane boundaries are important here for this twisted β-hairpin. Almost all opsins are further distinguished by four other conserved residues Y..E.....C..DY (in rhodopsin numbering Y178 E181 C187 D190 Y191) which presumably play the same role in both determined opsin structures. The conservation of the final tyrosine (often tryptophan) persists into GPCR such as tachykinin receptor with highest overall opsin blast scores, though it is not sufficiently universal to occur in the other three structurally determined GPCR.
Various studies pertain to the phenomenal conservation of these four non-cysteine loop residues. First, interactions with other proteins can be eliminated as these take place on the cytoplasmic side. Proper folding of newly synthesized opsin is a valid consideration. Y191 is proximal to the 9-methyl group of 11-cis retinal in the dark state; Y191A changes hydrolysis of the Schiff base, folded structure after photobleaching and chromophore release. R177 forms an ion pair with D190 but comparative genomics quickly shows this has narrow applicability.
E181 has been interpreted as the counterion in Meta I rhodopsin (displacing E113 in the dark state); the reorganization of EC2 then propagates to TM3 via a push from the disulfide. Yet it's hard to see how this could work universally because in LWS the residue is invariably histidine and in VAOP where it is always serine (as SK covarying anomaly). These residues lack the negative charge to offset the Schiff lysine. D and E are also found sporadically in non-opsin GPCR where counterion makes no apparent sense -- this could be pursued in the structure of ADRB1.
Almost all of the indels resolve to insertions despite their relative rarity compared to deletions proteomewide. These insertions evidently arose from some other mechanism than extension of splice donors or acceptors (indicated below by underlining) because the site of insertion is elsewhere, even allowing for gap uncertainties. If some sequence microstructure predisposed certain regions to insertions, little of that may remain 500 myr later. All the key developments in ciliary imaging opsins took place prior to lamprey divergence.
Overall, the pattern of insertions but not deletions suggest a structurally determined floor to admissible loop lengths. After discounting derived forms, all opsin classes other than neuropsins have the same minimal length, including cnidarian and ctenophore opsins. Thus the ancestral opsin likely had this length and pattern:
W.........P..GWS.Y..E.....C..DW........SY...........P.......Y
These region provides various synapamorphies for opsin classes. Each of these is represented by a single proxy sequence that accurately represents its ortholog class. For example, all UV7 arthropod opsins have the same two residue insertion at the end of TM4, not just the Rhodnius prolixis representative shown. Homoplasy can be seen in some instances (eg the five imaging cilopsins and the four dimly related neuropsins both have two extra residues following Y191) but it remains manageable when it occurs in two well-separated regions of what is a very deep and comprehensive gene tree.
Indels in other opsins
Informative indels would be very helpful in the peropsin/neuropsin/rgropsin group of opsins because their sequence relationships to ciliary and melanopsins are too weak to determine root topology. Note intron patterns, another class of even rarer genetic event and so even better suited for deep time scales, has already illuminated branching relationships to a certain extent.
(to be continued)
See also: Curated Sequences | Ancestral Introns | Cytoplasmic face | Ancestral Sequences | Alignment | Update Blog