Opsin evolution: key critters (ecdysozoa)
See also: Curated Sequences | Lophotrochozoa | Deuterostomes | Cnidaria | Update Blog
Key Critters: introduction to genome project opsins
Some species such as Drosophila have lost all ciliary opsins -- clearly this class of genes is not essential for a successful visually complex flying insect with 5-color vision, peripheral motion detection, polarized light capability and circadian rhythm (as one might have assumed from vertebrates).
Other protostome lineages such as the fruit nematode Caenorhabditis elegans function successfully without any vision at all, making this 'model organism' completely irrelevant to the evolutionary study of vision. However marine nematodes and a few others do contain highly diverged opsins (see below).
However bees, annelids, and mammals retain ciliary opsins so it follows -- pervasive, detailed convergence at the molecular level being impossible -- this must be the ancestral bilateran state state. In turn that suggests ciliary opsins in cnidaria and indeed that has been recently established in the lensing eye.
When the eye is reduced to a single pigment cell backing a single photoreceptor cell, the opsin of that species may be expressed only in one cell of the entire body. In this situation, the opsin may never show up in transcript collections, even with subtraction of common ones. One sees the importance of complete genomes here (versus transcripts or immunostained sections alone): absence of ciliary opsin evidence in a genome is truly evidence of ciliary opsin absence.
Vertebrates could never have evolved ciliary opsin vision had the bilateran ancestor possessed the limited opsin repertoire of fruit fly. Thus the most pressing question is -- assuming rhabdomeric opsins were thoroughly entrenched in the earliest bilateran imaging eyes and photoreception systems -- what kept ciliary opsins around in early bilatera? Recall early diverging deuterostomes (xenoturbellids, urchins, acorn worms, tunicates, and lancelets) lack imaging vision -- that emerged in full modern form on the lamprey stem.
Conversely, assuming cnidaria use ciliary opsins, what kept rhabdomeric opsins around so that they could later be co-opted by protostomes for their form of opsin-based vision? Evolution is strictly 'use it or lose it' over these time frames. Here cnidaria, or at least their larva, may also use rhabdomeric opsins. It seems that both classes of opsins have retained roles in most species, but very different classes were promoted to the imaging role in different branches of Bilatera. In fly, ciliary opsins have winked out; in nematode, both ciliary and rhabdomeric opsins are gone. While irrevocable, these losses would scarcely receive comment in non-model organisms.
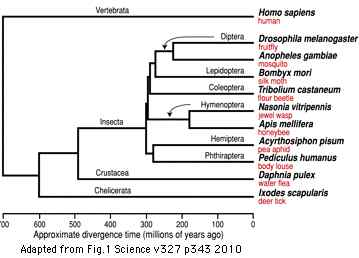
It's important to understand contemporary representatives of early diverging species (relative to the sequence of divergence nodes leading to human) are not archaic failed experiments nor primitive living fossils frozen in evolutionary time. Quite the contrary, all surviving extant species are equally successful and fully modern -- the tree of life is right-justified. Indeed their genes, regulatory signalling systems, and enzymes may be more finely honed than slowly evolving mammals because of more rapid evolution attributable to larger effective population sizes, reproductive mode, short generation time, and marine selective predatory pressures.
However we can still hope that ancestral character traits will still be reflected to some extent in these earlier diverging species and that with enough complete opsin repertoires from taxonomically appropriate species, ancestral genes and even whole visual systems can be reconstructed at key ancestral nodes on the phylogenetic tree. The story describing the evolution of the human eye then amounts to describing its status at these successive nodes with perhaps interpolative speculation between them. Definitely limits to knowledge exist because living metazoans provide only 35 nodes between sponge and human -- gaps between nodes may average 30 million years but can greatly exceed that (eg 135 myr between bird and platypus). This is offset by the occasional proposal for new deuterostome branches (Xenoturbella, Convoluta) or basal metazoan (Ctenophores.
The ideal set of genomes needed to study the evolution of the metazoan eye is only partly completed, underway, or not even proposed yet. In some cases, the genome size of clade representatives is so large (eg lungfish at 25x human) the species may never be sequenced, though satisfactory opsin transcripts could still be obtained. In others, the rate of evolution has been so fast so long that very little information about photoreception at ancestral nodes has been retained (eg the tunicate Oikopleura). Hagfish opsins, which would conveniently break up the crucial lamprey long branch, are not available at GenBank but here the animal has adopted a deep water habitat, meaning that its cone opsin genomic repertoire will be highly reduced, if not gone entirely, in its markedly degenerated eyes, though whatever remains of its opsins could still be informative.
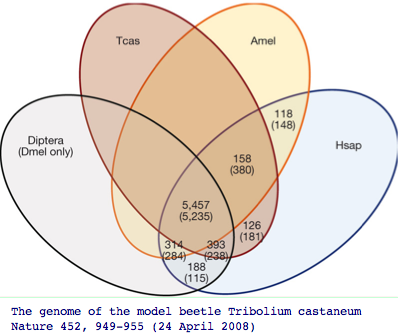
The impact of adding more genomes is to uncover more genes of the common bilateran ancestor that were masked by lineage-specific losses. Recall the beetle genome Tribolium uncovered 126 additional genes absent in other insect genomes but nonetheless present in human. Humans themselves of course have lost hundreds of genes even relative to the first land animal, so here too we need to pool mammalian and amniote gene pools to reconstruct that ancestor.
Model organism choices do not always coincide with genome sequence-ability, transcriptome projects, nor (worst of all) with more slow-evolving and less derived species. Finally, most sequencing speaks to narrow anthropocentric interests, whereas the sequencing need more broadly conceived is greatest farther back (to break up long branches). The evolution of the eye needs a rather different portfolio of genomes than a typical human disease gene because of the earlier intrinsic timing of the innovative events. In fact, one product of the investigation here is to spell out these needed genomes. Of course one obvious genome choice are cubomedusan jellyfish with their 24 eyes of 6 types.
It's worth reviewing genome status and recent experimental literature on key species. While abstracts are readily available at PubMed, access to free full text is unpredictable, so those links are collected when available. It suffices to reference only recent articles because those in turn cite the earlier literature and citation in turn of their paper are collected by Google Scholar (or AbstractsPlus at PubMed). Most opsin sequences in the Opsin evolution reference sequence collection have a PubMed accession as a field in their fasta header database; those can simply be compiled to an active link that opens all of them in one PubMed window.
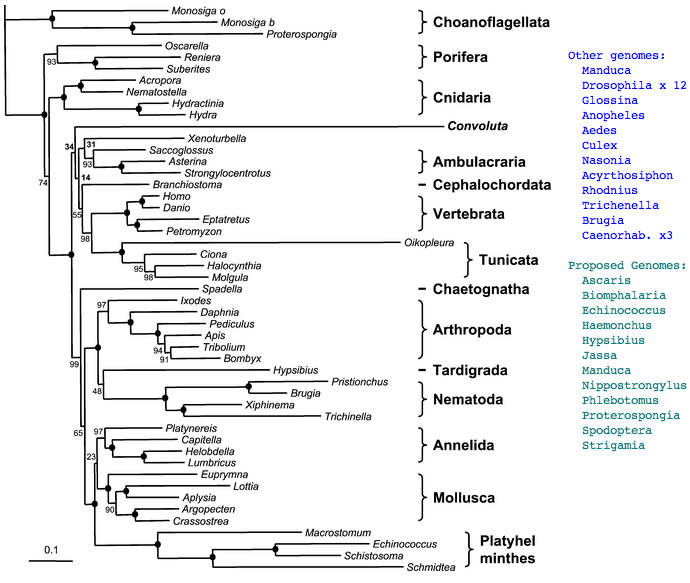
Figure adapted from: Acoel Flatworms Are Not Platyhelminthes: Evidence from Phylogenomics (H Philippe et al PLoS ONE. 2007 Aug 8)
Ecdysozoa: 101 opsins
This clade includes insects and other arthropods but not mollusks and annelids (lophotrochozoa). The focus here is on species with genome projects that allow complete opsin repertoires to be determined, as supplemented by annotation transfer from experimental species when 1:1 orthology can be established.
Genome projects have not sampled ecdysozoan phylogenetic diversity evenly to date but that may change as small genomes can be rapidly sequenced today. Studies of photoreception in non-genome species are limited by their inevitably incomplete repertoire of sequenced opsins and companion genes. Opsins in genomic species have determinable intron positions and phases and flanking genes so better prospects for inference of accurate descendent relationships.
An immense amount of experimental work on Drosophila melanogaster, recently reviewed from an evolutionary perspective, provides an excellent understanding of the evolutionary history underlying regulatory genetics, biochemistry, developmental and structural homologization of opsin expression across larval Bolwig organs and adult ocelli and eye.
While annotation transfer to the other 11 fruit fly genome projects is largely justified, that becomes problematic even across Insecta because of gene loss in drosophilids (notably all ciliary opsins), lineage-specific tandem expansion of opsin multiplicities and the necessary rationales for their retention, derived conditions, and better representation of ancestral characteristics in other species. It will prove very difficult even to get at ancestral dipteran vision starting from Drosophila. Yet species with simpler vision like Tribolium are no living fossils either, having lost opsins.
Imaging vision in ecdysozoa (and lophotrochozoa) is quite different from the chordate system, with rhabodomeric opsins residing in specialized microvilli rather than ciliary opsins in modified cilia. The signalling system and chromophore regeneration also represent substantial departures. At first there seems no common ground for a shared Ur-bilateran ancestor -- which signaling system was originally used for imaging vision and which lineage displaced it with the other? Some protostomes still utilize ciliary opsins in non-imaging photoreception and similarly some deuterostomes still utilize rhabodomeric opsins. Since the relevant opsin gene trees coalesce far earlier, this proves Ur-bilatera possessed both opsin classes (without clarifying which system was used for imaging vision, if either).
Blastp of any rhabdomeric opsin from any protostome against the set of all deuterostome opsins invariably gives vertebrate melanopsins as best match, whereas blastp of any protostome ciliary opsin (pteropsin) always has best match to TMTs (ancestral form of encephalopsin). That is, from the biomedical perspective, rhabdomeric opsins are just a clade-specific expansion of melanopsins largely irrelevant to human vision. Similarly invertebrate ciliary opsins not used in imaging vision primarily inform us on deeper ancestral origin issues. Note melanopsin and TMT are not orthologs at the level of Ur-bilateran nor even Ur-eumetazoan because gene duplication and divergence preceded the cnidarian last common ancestor.
The nature of vision at ancestral nodes has not yet been resolved, in part because pre-bilateran cnidaria photoreceptors studied so far as outgroup have been either ciliary, or based on distantly related cnidarian-specific opsins, or in the case of coral melanopsin, genomic sequence not yet associated with photoreception. In the Ur-eumetazoan common ancestor, this could imply ciliary opsin imaging vision, no imaging vision but convergent evolution (later independent invention) in the box jellyfish lineage, or even rhabdomeric imaging vision with subsequent displacement by ciliary opsins in cubomedusa and separately in later deuterostomes. Sponge larva presumably also utilize a ciliary opsin but here again it is unclear whether later metazoan use a system descendant from that.
It's sometimes asserted that imaging vision systems (all highly dissipative of ATP) were first enabled in the rapidly oxygenating Cambrian ocean, yet near-simultaneity is not a good fit to the arthropod fossil record (stalked eyes) nor molecular reconstructions. For example, extant representatives of early diverging deuterostomes (xenoturbella, acornworms, echinoderms, tunicates, amphioxus) all lack imaging vision (depending on how that is defined in scanning larva), so it seems clear that early arthropods had well-developed vision prior to the emergence of hagfish/lamprey. The majority of extant animal phyla have prospered for 540 myr without ever developing imaging vision.
Ecdysozoa .. opsin repertoire of the last common ancestor
Questions of ancestral opsin repertoires and their implied photoreception biology are best addressed after careful step-by-step reconstruction of opsin repertoires in each of the relevant lineages, exhausting available information in extant species rather than just add to a century of speculation. These reconstructions can help evaluate candidates for 'living fossils'. Thus the focus in this section is reconstruction of the opsin repertoire of just the last common ancestor of ecdysozoa. That can be combined later with parallel efforts on ancestral lophotrochozoa and deuterostomes opsins to get closer to the Ur-bilateran.
This program has already been set in motion with important recent reviews and new sequencing of opsins in arthropod outgroups to the much-sampled Insecta, providing new opsins from crustacean and chelicerates. The gene tree, as overlaid on clade divergence, shows color vision already well established prior to divergence of insects. However incoming data, in the form of a second distinct opsin from the ventral eye of horseshoe crab Limulus (a chelicerate, not crab (malacostracan crustacean)), already requires an earlier origin for the opsin class BcRh1 once thought specific to crustaceans.
Just as absence of effort on hagfish has needlessly delayed our understanding of chordate vision evolution, absence of effort on early diverging Ecdysozoa such as Pycnogonida (sea spiders), Myriapoda (millipedes), Onychophora (velvet worms), and Tardigrades (water bears) has seriously retarded reconstruction of the ancestral opsin repertoire in this lineage. Rather than yet another minor mammalian cone opsin or yet another butterfly gene expansion, biology is better served by sequencing more strategically placed species. Ironically the truly pivotal data may come out of genome projects rather than opsin research per se.
In classifying ecdysozoan opsins from a deeper evolutionary perspective, it is necessary to set aside narrow clade-specific expansions and contractions of opsin repertoires, however adaptively important to the individual species concerned. Wavelengths of peak adsorption -- subject to significant change from tuning residue substitution -- seem an unsound basis for evolutionary classification (though in retrospect work fairly well). This leaves phylogenetic alignment, signature residues and rare genomic events (such as indels and introns) as the main tools.
Here the remarkable observation in 2003 that a single lysine K90 (bovine rhodopsin numbering G90) suffices to define the phylogenetically valid class of ultraviolet opsins. Six years later, despite a vastly expanded data set, there is still near-perfect concordance of spectrophotometry, behavioral studies, alignment clustering, indel signature, intronic structure, and possession of lysine (rarely glutamate) at this position. This residue was previously known to be important to spectral tuning from bird C90S ultraviolet vision and human rhodopsin G90D night blindness.
This residue sits deep within transmembrane helix 2 as illustrated by a massive alignment of this region. That hydrophobic milieu is unworkable energetically for a charged residue unless a compensatory counterion exists. That negatively charged residue is presumably the ancestral counterion, negatively charged E171 (rather than the E113 of vertebrate ciliary opsins) or for positively charged residue, the Schiff base itself. K90, by taking E171 away from the Schiff base lysine K296, has the effect of leaving that protonated, an effect known to shift adsorption into the ultraviolet.
Observe however that opsins specialized to blue (not ultraviolet) are also satisfactorily classified in this same region (June 2009 current alignment below). These opsins have some other residue than lysine at 90 but share a one-residue deletion near the lysine that would shift its orientation relative to the chromophore as well as a proline six residues after the DRY motif, which is glycine in all other ecdysozoan opsins. This agrees with conventional phylogenetic alignment that sisters blue and ultraviolet opsins to the exclusion of long wavelength and blue-green opsins as well as to the more basal BcRh opsins operationally defined by clustering to two particular opsins from the crab Hemigrapsus sanguineus.
While K90 could potentially have arisen multiple times as the same solution to the problem of ultraviolet vision, the simultaneous presence of multiple other defining signatures render this improbable. Opsins with K90 thus date back to the common ancestor of chelicerates and insects (ie Arthropoda) if not earlier, though no such opsins are seen in lophotrochozoan whole genome projects (eg the mollusk Aplysia) or deuterostomes. Blue optimized opsins appear limited to insects. Consequently prior to gene duplication and divergence, the ancestral gene had K90, hence ultraviolet vision not tuned to blue.
This implies coalescence of the blue, ultra-violet and RH7-class ultraviolet opsins to a single gene prior to arthropod divergence. Similarly, despite many lineage-specific expansion, the long and middle wavelength opsins merge into a single gene in this same era. The same can be said for their ciliary opsins and those of deuterostomes. Since lophotrochozoa and deuterostomes possess but a single class of melanopsins -- notably with two introns identical to arthropod RH7 -- it follows that Ur-protostomes and Ur-bilaterans had but two opsins (subject to the caveat that ancestral gene duplication followed by gene loss in all extant lineages is undetectable). The Ur-bilateran did not have color vision but rather ocellar vision and circadian rhythm.
These two Ur-bilateran opsins had not yet coalesced because both classes are observed in earlier diverging cnidaria. However reconstruction of ancestral sequence for ciliary and melanopsin gene families at this node begins to exhibit a merger. That can be readily be seen using the opsin classifier because best-blastp of cnidarian melanopsin selects cnidarian ciliary opsins. Sponge larva presumably have at least one opsin; in the common ancestor, this may predate the gene duplication leading to the two opsins of eumetazoa. The status of neuropsins, peropsins and RGRopsins is unclear at most of these nodes -- their role may reside more in carotenoid metabolism and retinoid replenishment.
Panarthropoda: Hypsibius (water bear) .. 0 opsins
A 5x genome project for Hypsibius dujardini, a phylum of microscopic ecdysozoan was approved in July 2007 but Broad has not yet begun trace reads on the small 70 mbp genome (suggesting densely spaced genes with small introns as this is not likely highly derived). It could prove very useful for opsins as tardigrades are basal to all of Arthropoda and so shed light on that last common ancestor. In fact with accompanying centipede, horseshoe crab, amphipod, and priapulid genomes, the whole ecdysozoan ancestor will be accessible.
The only known fossil specimens are found in Siberian mid-Cambrian deposits and much later amber. The older fossils have three pairs of legs rather than four, a simplified head morphology, and no posterior head appendages and probably represent a stem group of extant tardigrades. Aysheaia from the Burgess Shale might be related to tardigrades.
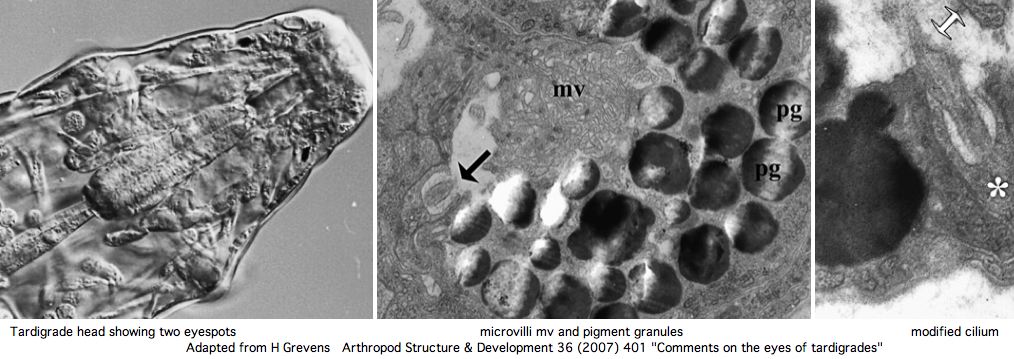
Nothing is currently known about photoreception or opsins in tardigrades -- barely that they have eyes. However a rhabdomeric opsin at the minimum may be expected in front of the pigment cups. However the current GSS and EST collections (about 6000 sequences) do not currently contain any convincing matches using various rhabdomeric and ciliary opsins as tblastn queries.
Greven has recently reviewed the situation in regards to tardigrade eyes. These consist of a pair of inverse pigment-cup ocelli located in the outer lobe of the brain. One (sometimes two microvillous (rhabdomeric) cells are the apparent photoreceptors, which are backed by a single pigment cup cell containing pigment granules (of unknown chemistry) in the outer dorsolateral lobe of the brain. Ciliary sensory cells located close by are probably epidermal mechano- and chemoreceptors rather than photoreceptors.
Phototaxis cannot necessarily be attributed to the ocelli prior to determination of the complete opsin repertoire of the tardigrade genome and its anatomical assignments. It is safe to predict however that the ocellus opsin here will classify as a basal melanopsin. A ciliary opsin, known to be present in tardigrade ancestor, may well be retained. Here the question is whether it is expressed in a ganglion perhaps homologously to those of Platynereis.
Panarthropoda: Onychophora (velvet worm) .. 0 opsins
The key arthropod outgroup Onychophora is also completely lacking in opsin data even though their eyes may provide important clues to the evolution of arthropod rhabdomeric vision -- a pair of simple ocelli at the base of the antennae on the first segment may be the ancestral visual design. The anatomy here consists of a chitinous ball lens, a cornea-like covering and a retina connected to the brain center via an optic nerve. Various Cambrian fossils look more or less like onychophorans, eg Aysheaia, but overall Onychophora do not support a Cambrian explosion.
G. Mayer makes the surprising observation that onychophoran eyes are innervated to the central (rather than lateral like ommatidia) part of the brain. More specifically, the posterior branch of the optic nerve connects to the posterior lamina of the central brain whereas the anterior branch, after bifurcating again, joins nerves connecting the antennal glomerulus to the mushroom body. Further, these everse eyes originate embyrologically from an ectodermal groove rather than the lateral proliferation zone of ommatidia which develop from lateral ectoderm of the ocular segment. Consequently ornychophoran eyes are better homologized to median ocelli of euarthropods than to their compound eyes.
Despite some historic confusion over cilia in onychophoran photoreceptors, the photoreceptors reside in microvilli and the ocelli are unambiguously rhabdomeric. However the presence of 9x2+0 cilia raises the question of whether the shift seen in deuterostomes is an abrupt discontinuous difference or less cosmic change just in intracellular targeting of gene expression and membrane ramification.
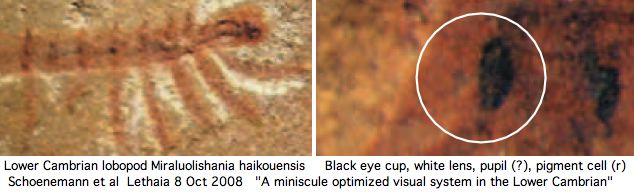
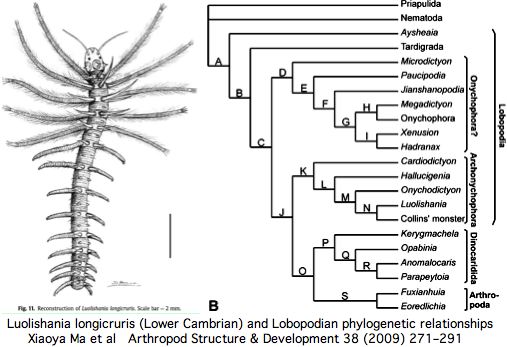
The number of these ocelli varies -- apparently because of lineage-specific structural duplications -- but the ancestral number, inferred to be two from extant lineages, has fossil support if the paired dark spots in the middle of the head in the Lower Cambrian species Luolishania longicruris (synonym: Miraluolishania haikouensis) are its only (lensed ocellar) photoreceptors. In this view, ommatidial eyes did not furnish the primary ancestral vision but are rather a dramatic later expansion of lateral photoreceptors within euarthropods.
This has implications for the opsins used in these respective photoreceptors and the evolution of this gene family. Here it will be important to determine the full repertoire of onychophoran opsins and where each is expressed. The hope here is that a stable association exists of opsin type with photoreceptor types, allowing more to be deduced about photoreception in the ancestral protostome and bilateran.
Recent Lower Cambrian lobopodian fossils from China have clarified the anatomy of these 543 myr old fossils and their phylogenetic relationships to living onychorphorans (which they closely resemble). The paired dark spots interpreted as [non-compound ocellar] eyes are quite small and positioned more dorsally than lateral (which has implication for central rather than lateral innervation). The light environment was bright (shallow marine).
While these fossils are probably not in the exact line of descent to any contemporary onycophoran, the last common ancestor is not far removed. They thus suggest that two symmetrically placed ocelli is the ancestral state and that these have a continuous homologous history without confusing gains and losses in photoreceptor structures or major brain re-wiring.
The question is whether these presumed ocelli also gave rise to compound eyes through structural splitting and subsequent specialization in descendent arthropod lineages. Conceivably the major optical system of arthropods evolved later from scratch (though recycling existing components and evo-devo regulatory modules). Another scenario is that these fossils -- and perhaps extant onychophorans -- had additional photoreceptors deployed without telltale pigment cups (making them effectively undetectable, as with ciliary opsins in protostomes). For example lateral photoreceptors providing roll orientation might have evolved into compound eyes with lateral brain connections.
While we will never know the sequence of the opsins utilized in these fossils, they are likely orthologous to the opsins in contemporary onychophorans, recalling the definition of orthology references the LCA and allows for lineage-specific gene duplications. Note however extant velvet worms are not exactly living fossils, being terrestrial animals of dark habitats, a shift accompanied many times by adaptive mutations in opsins. Perhaps methods of ancestral sequence reconstruction can adjust for this in some way.
Chelicerata: Ixodes scapularis (tick) 3 opsins
The genome project was completed long ago but has experienced a multi-year bottleneck in assembly release and publication. However contigs built from a subset of 19.4 million traces became available to tblastn of the GenBank "wgs" division by late 2007. Ixodes has a very conservative genome (regrettably 2.1 gbp in size), seemingly far less derived than drosophilids in matters such as intron, gene retention, and protein sequence conservation. This, in conjunction with the helpful phylogenetic position of chelicerate outgroup to the many insect genomes, has improved prospects for reconstructing the ancestral opsin repertoire of Arthropoda and eventually Protostomia and Ur-bilatera.
A large collection of annotated Ixodes ESTs is available at the DFCI Gene Index of which 3 are marked up (2 wrongly) as opsins. Using the Opsin Classifier, a full length gene could be recovered for the first of these (TC19272) on 24 Nov 07, intronated at the Trace Archives (4 introns, superb coverage), and added to the classifier fasta collection as LMS_ixoSca. The protein classifies with rhabdomeric opsins (ie with melanopsins) with a very respectable 57% maximal identity. The second and third intron have classical ancestral position (following GWSR and LAK) and phase (2 and 0). Synteny awaits assembly of large contigs -- adjacent exons are not spanned by single traces.
A fragment from a second melanopsin, found in June 2009, has the two exons and best blastp clearly diagnostic of an RH7-type UV opsin but has E in K90 position like some insect blue sensitive opsins. Assembly contigs are very short, ruling out synteny comparison and coverage is lacking for the first exon. There is no sign of additional UV or blue opsins.
On 15 Dec 2009, a peropsin probe from jumping spider became available. This allowed recovery of 4 exons (of 6 expected) from a likely orthologous peropsin from Ixodes and a predicted intronation of the spider sequence by homological transfer in which introns 1,3, and 6 are identical in position and phase to frog and amphioxus peropsins. These introns are universal to all peropsins, neuropsins, and rgropsins.
Thus the spider peropsin is not an isolated surviving gene -- more likely all of Chelicerata have a peropsin. The gene appears to have been lost on the insect-crustacean stem; once again, insects do not provide a satisfactory model organism system for ecdysozoan opsins. Sampling ever more of their genomes will not rectify this.
The Ixodes contigs are mostly small and disconnected relative to the exons; no adjacent gene information is present in the 6072 bp available after the stop codon according to blastx against GenBank nr. Thus synteny cannot be used to establish orthology to other peropsins.
No ciliary opsin is present in the current set of traces. Ixodes thus appears to have a fairly small repertoire of opsins. Note the pattern of loss of ciliary opsins was quite different than peropsin: the latter occurred once on a deep fundamental stem whereas ciliary opsin have been lost multiple times in arthropods and again in lophotrochozoans. This analysis assumes genomic coverage is complete (often not the case) and that sequences are not too rapidly evolving.
Ciliary opsin occurrence in protostomes + Daphnia Ecdy.Crust.Branc - Ixodes Ecdy.Arthr.Cheli + Daphnia Ecdy.Crust.Branc - Drosophila Ecdy.Insec.Dipte + Tribolium Ecdy.Insec.Coleo - Pediculus Ecdy.Insec.Phthi + Aedes Ecdy.Insec.Dipte - Nasonia Ecdy.Insec.Hymen + Anopheles Ecdy.Insec.Dipte - Manduca Ecdy.Insec.Lepid + Anopheles Ecdy.Insec.Dipte + Culex Ecdy.Insec.Dipte + Rhodnius Ecdy.Insec.Hemip + Acyrthosiphon Ecdy.Insec.Hemip - Capitella Loph.Annel.Polyc + Apis Ecdy.Insec.Hymen - Lottia Loph.Lopho.Mollu + Bombyx Ecdy.Insec.Lepid - Schmidtea Loph.Lopho.Platy + Heliothis Ecdy.Insec.Lepid - Schistosoma Loph.Lopho.Platy + Platynereis Loph.Annel.Polyc - Aplysia Loph.Mollu.Gastr + Platynereis Loph.Annel.Polyc - Helobdella Loph.Annel.Clite
>UV7_ixoSca Ixodes scapularis (tick) exon 1 missing, exon 2 disjunct, K90 is EIP 0 2 1 RRRIRSQANLLVFNLALSDLLMVLEIPLLVYNSLKLRPALGVW 1 2 GCQLYGLMGGLSGTSAIFSIAALSLERYLALGRPRDPFARLTRSRAFALSLSSWIYALCFSAWPLLGVTSPYVPEGFLTSCSFHFLSDATSDRCFVWIFFVAAWCVPLVFVTTCYSGILVTVIRSR KALAQES RRSELRVAKVSLALVLLWTVAWTPYAIVALLGITGRRNLLTPWGSMAPAMFCKSAAVLDPFVYGLSHPSFRRELAIMLPCLRPRQRPVSLTLRAVVQLPKRPGPRSAGSSTSVPVTAPGTTKDNHCPTPPNVSR* 0 >LWS_ixoSca Ixodes scapularis ocellar TC19272 UP|OPSO_LIMPO 0 MGSEGQRTNMSLLDELASPYMKNGTLVESVPDEMLYMVHPHWYNFKPMNPLWHSLLGFAMVILGVISVVGNSMVIYIMTTSKSLRSPTNMLVVNLAFSDW 2 1 CMMAFMMPTMAANCFAETWILGPFMCEVYGMVGSLFGCGSIWSMVMITLDRYNVIVRGVAAAPLTHKRAALMIFFVWFWALTWTLLPFFGWSR 2 1 YVPEGNMTSCTIDYLTKALWSASYVVAYAGGVYWTPLFINIYCYSKIVRAVAQHEKQLRLQARKMNVASLRANAEQTKTSAEARLAK 0 0 IALMTVGLWFMAWTPYLTIAWAGIFSDGSKLTPLATIWGSVFAKANACYNPIVYGISHPKYRAALARRFPSLVCMPPGGDQLDTRSEASGITTIEDKVMTTET* 0 >PER_ixoSca Ixodes scapularis (tick) XM_002409761 embryonated eggs XM_002435011 ABJB010816023 contig frags 62% ? 1 WLFGATGCQAYAFMGFLFGSAHIGTLTLLALDRYLATCRIGF 1 2 RSKPTFKRYFQLLLLVWLYGLFWAVMPLLGWAR 2 1 YGLEPSFQSCTIDWRHNDSSYKSFTLVYFVLGFLVPACIVLVCYRTSAIHIRAPKPKTVRRDVTDDYWASEEMVTV 0 0 MVALIVVTFFFAWTPYAVLCLWAVFADTKSVPHLLAMVPPLFAKTASTINPFIYFLSNPCIRADVLQLLGCRARSSPHMAISSDAVEEERCCQDQA* 0 >PER_hasAda Hasarius adansoni (spider) Deut.Chelic.Arachn AB525082 Go full 19960196 peropsin first ecdysozoan peropsin not yet in GenBank 0 MDDNMSEIALADDMSTLSTQEPSENVYPYVFPLSTHTIVGTYLIII 1 2 GILGTLGNGLVLVTFLRFRVLVTPTTLLLVNLAVSDLGLILFGFPFSASSSLSAK 2 1 WIFGEGGCQWYAFMGFLFGSAHIGTLALLALDRYLIACRISL 1 2 RGKLTFKRYTQMITVVWTYAFFWALMPLLGWGR 2 1 YGLEPSVTTCTIDWQHNDSSYKSFLIVYFVLGFMVPFAIIAVSYIAIARRVGKKSKERPVVRDLWTNERSVTL 0 0 MAFILIVTFFVAWSPYAVLCLWTIFAEPNTVPPFLTLIPPLFAKSSTVVNPLIYFLSNPKLRTAILSTLSCCNEAPIQNIELPDSPERAANNDAI* 0
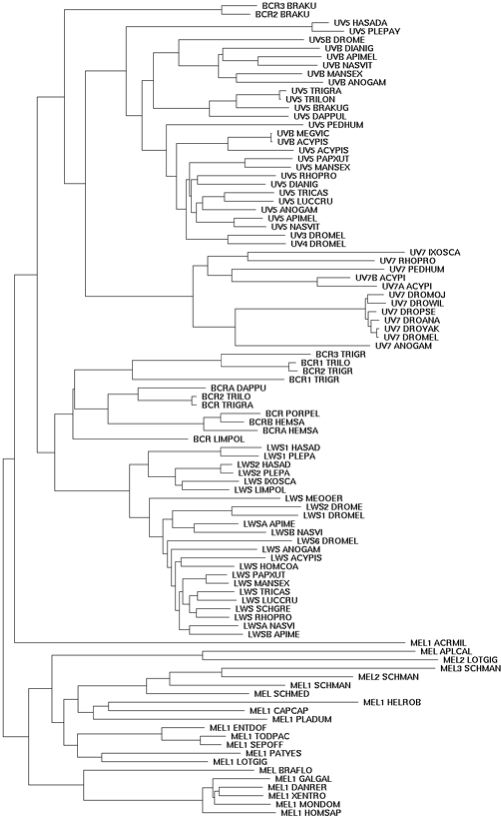
The 11 chelicaterate opsins available in December 2009 (after consolidating nuisance GenBank entries for Limulus): BCR_limPol Limulus polyphemus (horseshoe_crab) opsin 5 FJ791252 ventral eye LMS_limPol Limulus polyphemus (horseshoe_crab) CRBOPSINA L03781 lateral eye and ocellus (5 aa variant) LMS1_hasAda Hasarius adansoni (jumping_spider) HaRh1 kumopsin1 AB251846 LMS2_hasAda Hasarius adansoni (jumping_spider) HaRh2 kumopsin2 AB251847 LMS1_plePay Plexippus paykulli (jumping_spider) PpRh1 kumopsin1 AB251849 LMS2_plePay Plexippus paykulli (jumping_spider) PpRh2 kumopsin2 AB251850 LMS_ixoSca Ixodes scapularis (tick) P35361 ocellus LMS_loxLae Loxosceles laeta (spider) fragment EY188471 venom gland UVK_hasAda Hasarius adansoni (jumping_spider) HaRh3 kumopsin3 AB251848 UVK_plePay Plexippus paykulli (jumping_spider) PpRh3 kumopsin3 AB251851 UV7_ixoSca Ixodes scapularis (tick) contigs PER_ixoSca Ixodes scapularis (tick) contigs PER_hasAda Hasarius adansoni (jumping spider) AB525082
The phylogenetic arrangement of these species is (Limulus,(Ixodes,(Loxosceles,(Hasarius,Plexippus)))). Molecular clock dating of divergences (late Paleozoic just for land chelicerates) is under some dispute.
This leaves Pycnogonida (sea spiders) the last major unrepresented chelicerate group (even more basal if chelifore appendages aren't homologous to true chelicerae, in conflict with Hox expression boundaries showing anterior-most appendages also deutocerebral). Shallow water species have two pairs of dorsally located eyes. Given that the body is generally just a millimeter or two, these eyes are small and quite simple. A longwinded 1891 dissertation on their larval and adult anatomy is available as well as a 1973 ultrastructural and modern account.
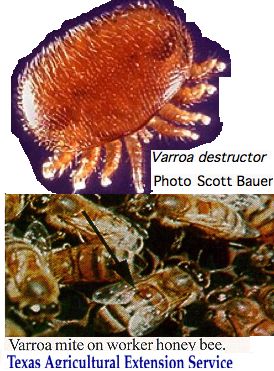
Chelicerata: Varroa destructor (bee mite) 1 opsin
The study of opsin evolution in arthropods must take crumbs from the table -- genomes sequenced from other species for other reasons. To date, species have not been selected for strategic phylogenetic position or for retention of ancestral characters. The Varroa genome illustrates this:
Contigs of 1-2 kbp size from this species were deposited to GenBank on 11 Jan 2010. The accompanying article, entitled "Genome sequence survey of the ectoparasitic mite Varroa destructor, a major pest of the honey bee" has not yet appeared. This genome -- while a welcome addition to Chelicerata -- proves very disappointing in terms of opsins.
Only a single exon of one opsin can be recovered with any confidence, despite a great variety of chelicerate probes used as tblastn queries. It has the K296 Schiff lysine and intron position and phase consistent with ecdysozoan peropsins though terminates too early to complete the usual motif. No other exons can be located in this contig even though adequate base pairs remain. Another exon with plausible splice junctions and middling blast score can be located in another contig but cannot be said for certain to originate from an opsin.
Consequently it is difficult to say whether genomic coverage is low (said 8x via 454), the assembly faulty, or the species very depleted in its opsin repertoire. No transcripts or gene models are currently available.
It is not clear how much of a role vision would play in the life cycle of this species. The female mites live outside on the bee, tucked inside an abdominal fold feeding on the hemolymph via a hole in the exoskeleton. In capped hive brood cells, the mite lays eggs that feed off larva and leave with them, already mated, as the bees become adults. Consequently varroa mites never need to navigate the environment on their own -- they are always a passenger of the bee which is well-equipped with opsins.
Eyespots are not evident in photos and have not been discussed in the literature. It would not be unprecedented for an organism to have lost all opsins -- consider nematodes.
>PER_varDes Varroa destructor ADDG01041468 0 TSLLIVTSFLSTWTPYAALCLWSVFFGPSTVPSWLMIAPNICAKASGALNPFIYSLSNR 2 2 GIVGLIANFVVLGMFYRTPGRLSPSSMVLLNLTVSDICILLLGFPIHTVVNFAGR 2
Crustacea: Daphnia pulex (water flea) .. 7+ opsins
An 8.7x genome assembly was released in July 2007 at JGI with further support at wFleaBase. A May 2009 meeting report suggests an imminent release of initial publications by the 370-member consortium.
The gene count, supposedly 39,000, may be inflated with genomic transcript noise that does not really code for protein, contig assembly errors resulting from polymorphism and use of paired end reads and over-counting of gene fragments and recent processed pseudogenes. JGI and Gnomon models to date err grievously on ciliary opsin gene models because they lack the last exon (below) which is necessary to complete the covalent lysine motif to FR.
This crustacean, basal to Hexapoda arthropods, provides a potentially important outgroup to insects (together forming Pancrustacea). However the opsin story, summarized in a meeting abstract is an embarrassment of riches, not conducive to deducing ancestral arthropod genome content. The total number of opsin genes came in at 37, comprised of 22 rhabdomeric opsins (mostly long wavelength), 7 ciliary opsins (pteropsins), and 8 in a novel family without close affiliates. A post on the Ixodes list serve even raises this to 46 by Feb 2008.
This seems excessive given Daphnia has a single medial compound eye with merely 22 ommatidia with 8 photoreceptors each, an under-focusing lens, and a three-ocellus naupliar eye, yet circadian rhythms and a need to assess water turbidity, depth, and distance from shore. Daphnia also can detect polarized light. It's not clear that exquisite color discrimination potentially afforded by dozens of opsins would be advantageous for a 22-pixel array; experimentally, only four wavelengths of peak sensitivity are observed at 348 (UV), 434, 525, and 608 nm in dorsal ommatidia.
Again the possibility arises that K-rhodopsin gene duplicates could have taken on other sensory or metabolic roles (digestion of complex algal carotenoids). Planned in situ hybridization studies may illuminate biological roles of these opsins. The pteropsins are probably of most interest from the ur-bilateran perspective.
Gene models have not been submitted yet to GenBank but are extractable by text query at wFleaBase. What is needed here however is not the clutter of 37 sequences but their collapse into UV, blue, long, pteropsin, and novel ancestral representatives. This would remove the noise from lineage-specific expansions. The intron structure could provide very important support to classification schemes.
To a certain extent, this has been accomplished by June 2009 at FleaBase as text searching by 'opsin' turns up 25 matches, many in tandem pair sets (which could reflect assembly error to some extent). There is no explanation of how 37 opsins got expanded to 46 then reduced to 25 with ciliary and novel opsins no longer not listed. Despite assigned accessions, no gnomon gene models have been released at NCBI.
Intronated gene models can be manually extracted from scaffold dna (done for four below). These models, taken at face value, unsurprisingly have best-blast at GenBank to Triops and other crustaceans (20 non-Daphnia opsins, all melanopsins), which mercifully have been analyzed in a careful Feb 2009 paper. This study considered only non-EST Branchiopoda (like Daphnia) and Malacostraca melanopsin sequences that likely under-represent opsin evolutionary information available from the full seven classes of Crustacea.
The only Daphnia opsin (NCBI_GNO_472553) with a transcript (FE295533) has been assigned to the BCRH1 group (middle wavelength MWS). One Daphnia opsin has a lysine at position K90 (bovine rod rhodopsin numbering) considered proof of UV purposing.
The value of Daphnia genomic opsins relative to other crustaceans lies in their intronation, which distinguishes expansions arising through retroprocessing from tandem and segmental duplication of a few master intronated genes (which would then be the orthologs to other arthropod opsins).
Indeed the intronation pattern -- typically far more deeply conserved than protein sequence -- could link pteropsins more convincingly to lophotrochozoan and deuterostome opsins than alignments with percent identities in the 20's. However, in comparison to Apis opsin counterparts, Daphnia has experienced numerous intron gains and losses, not furnishing a good guide to the ancestral state.
NCBI_GNO_176434 scaffold_53:626704-628972 Blue opsin [probable ortholog of Triops longicaudatus RhC] NCBI_GNO_416624 scaffold_95:369266-373273 Opsin Rh3 Inner R7 photoreceptor cells opsin NCBI_GNO_366144 scaffold_14:844292-847788 Melanopsin NCBI_GNO_557324 scaffold_2568:2224-6662 Short wavelength-sensitive opsin [defective model fragment but KMAACVDPFVYAINHPKYR] NCBI_GNO_750363 scaffold_40:707906-709794 Compound eye opsin BCRH1 (brachyuran crab RH1) NCBI_GNO_754363 scaffold_40:716143-718346 Compound eye opsin BCRH2 (brachyuran crab RH2) ... (rest are BCRH1 and BCRH2 types) >UVV1_dapPul Daphnia pulex NCBI_GNO_176434 FE384049 EST 53% identical Apis mellifera 69% Triops RhC 0 MLGWNTPEDYMSYVHP 21 YWKTFEAPNPFLLYMIGFLYTIFMFCCVAGNGVVIWIFTN 2 1 CKSLRTPSNMLVVNLAILDMLMMLKSPVMIINSYNEGPIWGKLGCDVFGLMGSYNGIGSAVNNAAIAYDRHR 2 1 TISRPLDGKLSRKQVTLMIVAIWAWATPFSVMPFLGIWGRYVP 1 2 EGFLTTCTFDYMTEDASTRFFVGSIFVYSYVIPLAMLIFYYSKIVRSVGDHEKTLRDQAKKMNVTSLRSNRDQNEKSAEVRIAKVAIALATLFVFAWTPYAFVALTAAFGNR 2 1 SVLTPLLSTVPACCCKLVSCINPWIYAINHPRYR 2 1 MELQKKMPWFCIHEPVPTNDDSSVGSATTEMSGVSKETSS* 0 >UVV2_dapPul 49% penultimate intron lost, last intron has slid back 2 aa 0 MNGWNTPADYKSYVHPHWLSYEEPNPMLHHLLGVLYIFFMIASCLGNGIVIYIFST 2 1 TKELKTPSNILILNLAICDFIMMIKTPIFIVNSFNEGPVFGRLGCSIFGLLGAYVGPCSAVTNAAIAYDRYR 2 1 CISDPMGKRWSKSQASLIVLGCWVYASPVSLLPFTEIVNRFVP 1 2 EGYLTSCTFDYMTDNLETKMFVFILWIWCWIMPLGVIIFSYGKITTQVMTHEARLKEQAKKMNVESLRSGANKDARNEIRVAKVGISLTTLFLLSWTPYFAIAFIGCYGNR SLLTPGLSMIPACTCKMAACVDPFVYAINHPK 2 1 YRLELMKRFPWLCVHEKDDSTRSENSTNATIASEAESRT* 0 >BCRa_dapPul Daphnia pulex NCBI_GNO_149114 53% identical MWS_hemSan, 72% Triops longicaudatus RhA AB293433 0 MSNNLSSGYSSVAYRSEGASVLWGYPPGLSIVDLVPDDMKEFIHPHWNKFPPVNPMWHYL 21 LGVIYVILGITSVT 1 2 GNSLVVHLFAKTRDLRTPANMFVINLAFSDLCMMITQFPMFVFNCFNGGVWLFGPLFCELYACTGSIFGLCSICTMAAISYDRYNVIVNGMNRRRMTY 1 2 GRAGGLILFCWIYAIGWSIPPFVGWGKYIPEGILDSCSFDYLTRDTM 0 0 TISFTCCLFAFDYCVPLIIIIFCYYHIVRAIVHHEDALRDQAKKMNVSSLRSNADQKSQSAEIRVAKIAMMNITLWVAAWTPYAAICLQGAVGNQDKITPLVTILPALIAKSASIFNPVVYAISHPKYRL 0 0 ALQKALPWFCIHEKEEKEPPQDRREDSQSIATTNTNSSDVSLP* 0 >MEL1_dapPul Daphnia pulex NCBI_GNO_366144 no close homologs 0 MTSSNDSAGYLWAINATIWIIDDSNETLGIDWDDWDVSLWTQEQRQLLEHGGIPRQVHVALGVLLSFIVLFGFAANSTILYVFSR 2 1 FKRLRTPANVFIINLTICDFLACCLHPLAVYSAFRGRWSFGQT 1 2 GCNWYGMGVAFFGLNSIVTLSAIACERYIVITSSSCRPVVAKWRITRRQAQK 0V 0 VCAGIWLHCAALVSPPLLFGWSSYLPEGVLVTCSWDYTSRTLSNRLYYFYLLFFGFFLPVSVLTFCYAAIFRFILRSSKEITRLIMTSDGTTSFSKSTVSFRKRRRQTDVRTALI ILSLAILCFTAWTPYTIVSLIGQFGPVDEDGELKLSPMVTSIPAFLAKTAIVFDPLVYGFSSPQFRNSVRQILRQQSISSSGNAGNRAGPNNMAMARTAIQNSRASSHATVSSF SRNARMFPKDPLSKKTPNDPFVSTPLAVQQIPHFRLPTDVDINEQQFRRGIYANKSVSYWIDIIVLLQLGENLRKSCMKRKNSFKIPAGSIPQKNKLSNSRCSLLEDVSTHSLA LRQMIFRKEGELYLFHHQPSHNAELAANKMDHQGNNKRIRRRFSEADMMHRSGKCRKNLPVSTSFDQ* 0 Daphnia opsins have no experimental data but their 'best-blast to PubMed' allows inference from opsins with experimental data: UVV1_dapPul NCBI_GNO_416624 ... 43% Acyrthosiphon pisum rhodopsin 7 XM_001944891 BCRH1_dapPul NCBI_GNO_149114 ... 72% Triops longicaudatus AB293433 MEL1_dapPul NCBI_GNO_366144 ... 33% Patinopecten yessoensis scop1 Gq AB006454 TMTa_dapPul ... 36% Apis mellifera pteropsin TMTb_dapPul ... 36% Apis mellifera pteropsin
This blast twilight zone is especially dangerous for photoreceptor opsins because they are embedded in much larger gene family of generic rhodopsin and GPCR which share many structural and signaling properties. A slowly evolving generic rhodopsin might well score higher than fast evolving photoreceptor opsins. Gene expansions are noted for markedly enhanced rates as copies neo- or subfunctionalize. The generic rhodopsin might also share diagnostic residues through convergence at least at the level of statistical significance ambiguity. Consequently intron location/phase and synteny can provide important backup.
The synteny circle surviving at this phylogenetic depth will be local (optimistically Pancrustacean). That is, the blue opsin of Daphnia might in synteny with Drosophila (ie establish orthology) but not to Platynereis ciliary opsin much less any vertebrate opsin (eg encephalopsin). This could be remedied to some extent by ancestral gene order reconstruction. The degree to which synteny can contribute to validating orthology relations within opsins is not currently known.
Ciliary opsins for Daphnia, absent from the collection of 25 pipeline-labeled genes, can be located by querying with Anopheles counterpart. Stored at the Opsin Classifier as TMT_dapPul, these are plausibly orthologs of deuterostome and lophotrochozoan ciliary opsins, as are new ciliary opsins from Culex, Aedes, Tribolium, and Bombyx. Counterparts to this gene and presumably its associated photoreceptor structure are missing in Drosophila, Nasonia, and other genomes.
In Daphnia, with its high level of apparent tandem duplication and 'excess' of opsins, the opsin of each class with highest external blastp score may be the parental gene and best conserve the function observed in its counterpart in other species.
>TMTa_dapPul Daphnia pulex (water_flea) last exon uncertain 45% id TMT1_anoGam 0 MPVWVYWSASAYLLFISIAGLFMNIVVVVIILNDSQ 0 0 KMTPLNWMLLNLACSDGAIAGFG 2 1 TPISAAAALKFTWPFSHELCVAYAMIMSTA 1 2 GIGSITTLTVLALWRCQHVVWCPTNRNSNFTDPNGRLDRRQGALLLTFIWTYTLIVTCPPLFGWGRYDREAAHIS 2 1 CSVNWESKMDNNRSYILYMFAMGLFIPLMAIFVSYISILLFIHK 0 0 SQQTSNNSDTVEKRVTFMVAVMIGAFLTAWTPYSIMALVETFTGDNVTNDSVSSEIKFYAGTISPAVATVPSLFAKTSAVLNPLIYGLLNTQ 0 0 FRTAWEKFSSRFLGRKKRHQRSQMAMGVSHKRRRDYLRTLLNRPASDEPAIVQHPSTKEMASSQAVSCVVVSNLDVPRAPNNSYVTVNDE* 0 >TMTb_dapPul Daphnia pulex (water_flea) ciliary long tail 60% identity 0 MPTWAYRLTAAYLLLISVLGLIMNVVVVIVILNDSQ 0 0 RMTPLNWMLLNLACSDGAIAGFG 2 1 TPISTAAALEFGWPFSQELCVAYAMIMSTA 0 0 GIGSITTLTALAIWRCQLVVCCPAKRKSAFTNHSGRLGCRQGVILLVIIWIYALAITCPPLFGWGRYDREAAHIs 2 1 CSVNWESKTNNNRSYILYMFCMGLVVPLAVIIISYVRILRVVQK 0 0 NQQQSGNVHRHRRDAAEKRVTMMVACMIAAFMAAWTPYSILALFETFIGQDNHSTYYSSRINNATNFSSAFPDGDLSYVGTISPAFATIPSLFAKTSAVLNPLIYGLLNTQ 0 0 FRLAWERFSLRFLGRFQCHRTQGVSGQHGANHHKTRRNVRKYLPNCYGDSRSLKPTPTVHLPMKEMVVSHAEQKVKTAQEQASSSVTKITTIPLISSDNQTIVSCPSSIMAN CQQHETNQANHQQAARPDKVVDHQHLLQPNRLSSLLSLSLPSVLISTPNLPCSAQRQSAAEDQAMATCQQMTSGRIRDQQQQSDSFVVVGLLSRSADCYHHHTGDVEQFVFLDSTVDELGLTARSASP* 0
Hexapoda: Tribolium castaneum (flour beetle) .. 3 opsins
The red flour beetle, which is highly dark-adapted in lifestyle, has lost its blue opsin but not ultraviolet according to both the newly published genome project and specialized experimental querying, retaining the long wavelength ancestral color vision opsins and ciliary opsin (which is called pteropsin in insects though likely a strict ortholog of vertebrate TMT). The Tribolium genome article 110 page supplemental contains an excellent Table S14 of all known genes involved in insect eye development.
The fellow orthopteran, the corn rootborer Diabrotica, furnishes an ultraviolet opsin
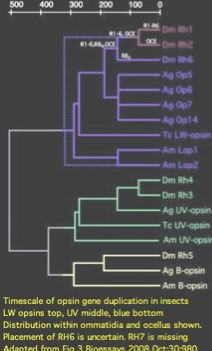
Insect opsins are expressed non-uniformly across individual eye units (ommatidia) within compound eyes. In Drosophila, six peripheral photoreceptor cells R1-R6 express LW opsin which detect brightness, projecting into the upper optic neuropil (lamina). Central photoreceptors R7 and R8 provide color vision via UV, blue, and LW opsins that project into the second (medulla). The dorsal rim area ommatidia are modified to detect polarized light.
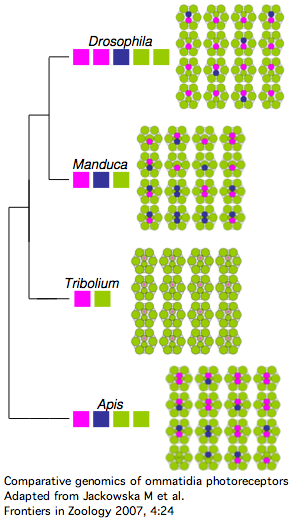
The comparative genomics of ommatidia number and opsin utilization is indicated in the figure. Opsin gene loss raises different issues, namely replacement, from the more familiar gene gain issues (differential rewiring). After discussing various sequential mutational scenarios and the necessity of each step being adaptive or at least near-neutral, Jackowska et al settle upon expansion of LW opsin expression into all photoreceptor cells, resulting in co- expression with blue opsin in some R8 cells and UV-opsin in R7 cells. This is followed by loss of expression or pseudogenization of blue opsin. Although co-expression defeats the purpose (via spectral summation) of separate opsins that enable color vision, there are precedents in butterflies and (typically nocturnal) vertebrates.
It's also known how Apis and Manduca (also genome project species) end up with nine photoreceptor cells per ommatium instead of eight -- it's due to duplication of R7 cell fate (across all ommatidia). That raises the interesting question of whether such cell duplication simply results in duplication of opsin expression at the molecular level. That's not the quite the case today because the two central R7-like cells exhibit differential opsin expression. It's not known whether additional mutations were needed to attain this.
In summary, insect genomes are fairly straightforward in terms of their contribution to establishing the ancestral arthropod visual system, but their real value lies in the extensive comparative data available within Insecta, ecological studies of adaptive vision, and the experimental genetic opportunities within Drosphila (eg a recent article exploring deviations from ommatidia expressing but a single opsin). However no single insect genome can serve all purposes because of gene loss (eg ciliary opsins in Drosophila).
That's also the case for non-opsin GPCR which have gained a new importance given the possibly paraphyly of the opsin gene tree (ie some opsin gene duplicates may have given up retinal to signal via other agonists). Here we are fortunate to have a genome-wide inventory of neurohormone GPCRs in Tribolium. This turns up 20 biogenic amine GPCR (21 in Drosophila, 19 in bee), 48 neuropeptide GPCR (45 in Drosophila,35 in honey bee), and 4 protein hormone GPCRs (4 in Drosophila, 2 in bee) with likely ligands for 45 of the 72 Tribolium GPCR. The flour beetle retains an ancestral vasopressin GPCR and cognate peptide unlike other studied insects which are not adapted to such an extremely dry environment. On the other hand, Tribolium lacks allatostatin-A, kinin, and corazonin. This covers comparative genomics of 340 million years of insect GPCR evolution -- it is very common for new agonist/receptor couples to arise and old ones to disappear. Again we see genome density sampling will need to be high to sort out Ur-bilatera.
>UV5_triCas Tribolium castaneum (flour_beetle) 0 MYVVHPFKIIRNKVTILRTMETMANHLGWNVPKDELIHIPQHWLVYPEPEASMHFLLALIYIGFFIMATIGNGLVIWIFST 2 1 SKSLRTASNMFVVNLAICDFAMMIKTPIFIYNSFYRGFALGHLGCQIFAFIGSLSGIGAGMTNACIAYDRYT TITRPFDGKITRTKALVMIIFVWGYTIPWAVMPLLEIWGRFAP 1 2 EGFLTACSFDYLTDTFDNHMFVTSIFICSYVIPMSMIIYFYSQIVSKVFSHEKALREQ 0 0 AKKMNVESLRSNQSQQASQSAELRIAKAAIAICSLFVASWTPYAVLALIGAFGDQSLLTPGVTMVPACACKFVACLDPYVYAISHPKYR 2 1 LELQKRLPWLAIKETAASETQSTTTENTTTQSATTTT* 0 >LWS_triCas Tribolium castaneum (red flour beetle) ES544655 3 exons from AAJJ01000967 5 fusion relative to bee 0 MSVMGEPNFIAWAAQRSGYGGGNLTVVDKVLPDMLHLVDAHWYQFPPMNPLWHGILGFVIGVLGFVSIVGNGMVIYIFSSTKALRTPSNLL VVNLAFSDFLMMlCMSPAMVINCYNETWVLGPLVCELYGMSGSLFGCASIWTMTFIALDRYNVIVKGLSAQPLTKKGAMLRILIIWVFSTLW TIAPFFGWNRYVPEGNMTACGTDYLTKDWVSRSYILVYAVWVYFVPLFTIIYSYWFIVQ 0 0 AVAAHEKSMREQAKKMNVASLRSSEAAQTSAECKLAKIALMTITLWFFAWTPYLVTNFTGIFEGAKISPLATIWCSLFAKANAVYNPIVYGIS 2 1 HPKYRQALQKKFPSLVCAGEPDDTTSTASGVTNVTTDEKPATA* 0 >TMT_triCas Tribolium castaneum (60%)55 298 encephalopsin-class ciliary 0 MKNFNSTEIGDELLIPVEGYIAAAVVLFCIGFFGFSLNLTVIIFMLKERQ 0 0 LWSPLNIILFNLVVSDFLVSVLGNPWTFFSAINYGWIFGETGCTIYGFIMSLL 1 2 SITSITTLTVLAFERYLLIARPFRNNALNFHSAALSVFSIWLYSLSLTIPPLIGWGEYVHEAANLS 2 1 CSVNWEEKSPNSTSYILYLFAFGLFLPLVIITFSYVNIILTMRR 0 0 NAAFRVGQVSKAENKVAYMIFIMIIAFLTAWSPYAIMALIVQFGDAALVTPGMAVIPALLAKSSICYNPVIYIGLNAQVKGAKWVSGLIYLFQFQQAWMQKWKKNRR GSDALGTSRVMLETIHQACRDEKTDKLLEKKTKFCKDFETDVSML* 0
Hexapoda: Pediculus humanus (louse) .. 3 opsins
The body louse genome, being favorably small at 108 Mbp, is well along with 2.2 million traces and a contig assembly disentangled from its endosymbiont bacterium. An excellent genome article appeared in June 2010 in PNAS. Sequencing is medically motivated. The lifestyle of this hemimetabolous (nymph-like adult, no pupal stage) insect does not suggest a full spectrum of metazoan photoreceptors; indeed we shall find but 3 opsins. Even that seems a lot for a single lateral ocellus of 130 rhabdomeric photoreceptor cells lacking Semper and dedicated pigment cells. The broader interest here is intronation and synteny of these opsins (hence orthology), not available in many insects with opsin studies. It requires quite dense sampling to get ancestral introns for each arthropod opsin class because high rates of intron gain and loss can occur.
I reconstructed 3 multi-exon louse opsin genes on 24 Dec 07 by tblastn of numerous queries against GenBank wgs database division. These apparent rhabdomeric imaging opsins are stored in the Opsin Classifier as INSE_LWS_pedHum, INSE_UVV1_pedHum, and INSE_UVV2_pedHum. Louse otherwise seems a gene loss story in terms of relic ciliary opsins or even melanopsins so not especially favorable for retention of ancestral characters. The new opsins potentially provide trichromatic color vision to the louse in the short, blue, and long wavelength photoreception regimes, though lambda max awaits experimentation as the second ultraviolet opsin could be either re-tuned or co-opted for some other function, as in bumblebee where a UV opsin is expressed in proximal lamina rim, antennal lobe, central complex and protocerebrum clusters. That seems likely because INSE_UVV2_pedHum is back to ancestral tyrosine in (bovine rhodopsin) position E113 whereas true ultraviolet insect opsins all specify phenylalanine here (which relaxes lambda max into the ultraviolet, ie closer to that of free retinal).
CA Hill of the louse genome annotation team discussed 3 opsins back in a June 2007 email session, calling PHUM001073 perhaps an ultraviolet opsin while rejecting a fourth PHUM000074. These gene models are not released to GenBank nor is that terminology used in the meagre search capabilities of P. humanus VectorBase. Upon whole proteome file download, PHUM001073-RA turns out to be an unintronated dna fragment matching residue 44 to stop codon of INSE_UVV1_pedHum. PHUM000074-RA has nothing to do with opsins. PHUM005795-RA is missing the first 49 residues of INSE_LWS_pedHum but otherwise identical. PHUM001044-RA is a fragment beginning at residue 55 of INSE_UVV2_pedHum. In short, it's hard to find full length genes without benefit of the Opsin Classifier, cdna, or ab initio gene predictor.
Hexapoda: Rhodnius prolixus (kissing_bug) .. 4 opsins
Yet another genome project completed long ago at the trace level but sitting around unassembled until 17 June 2009 (tblastn now at GenBank wgs). In August 2008 some 6,879,098 trace reads and 16,284 EST sequences were available. This number of traces is more than adequate for a good assembly but until now, opsins had to be fished out by exon by exon using blastn of trace archives.
Rhodnius prolixus, a large blood-sucking hemipteran insect that is carrier for a parasitic protozoan (Trypanosoma cruzi) responsible for Chagas disease through bites around the eyes and mouth. Chagas disease is a currently incurable tropical disease that damages the heart and nervous system. Rhodnius is nocturnal, with possible implications for its opsin repertoire, but becomes active at night. It is found in South and Central America, primarily in domesticated rural areas, currently affecting 16-18 million people and killing around 20,000 people annually. Darwin is sometimes claimed to have suffered from Chagas disease as a result of a bite (implausibly in northern Argentina) reported in Voyage of the Beagle diaries.
Rhodnius clearly has three distinct melanopsins and a ciliary pteropsin. One is a long wavelength sensitive gene most closely related (84% identity) to Tribolium but whose intronation pattern is closest to Apis (a phase 00 intron is missing in Rhodnius). The other two Rhodnius melanopsins have K90 so adsorption in the UV. The ciliary opsin is closest to that of mosquito and flour beetle but quite diverged at 56% identity.
>UV7_rhoPro Rhodnius prolixus (kissing_bug) Pterygota K90 at KMP, ortholog RH7 of droMel 0 mKYFHLYPIEQWKMHRFFTEEYLKLVNTHWFEYPPPNKQIHYIFAAVYFLVMLVGVSGNLLVIFMILR 2 1 FRTLRTSSNILILNLAVSDFLMVAKMPVFIYNSFYFGPVLGEM 1 2 GCHFYGFIGGLSGTASILTLAAIAMDRYLGIAHPLNFNQGRAKKRTIVWITFIWVYSITFASIPLSHIGVKTYVPEGFLTSCSFDYLSTDIQNRCFIFIYFVAAWCLPLLVIITSYVGICREVLRVSLIRKGQE REQRKREAKLSAILALATFLWFLSWTPYAAVALLGIFGYKNHITQLASMIPALFCKTAACVNPFIYGLNHPRLRQQLLKLCCKKRYNLEKTHFSRSWRNTSCSFKLKEQSLCNVSQSRLRRTSTVASEPSEHSTHFM* 0 >UV5_rhoPro Rhodnius prolixus (kissing_bug) exon 1 missing, K90 at KTP 0 0 1 ASTSGNIRTLGWNLSPEDLKHIPEHWLSYPEPEPILNYALGVLYIFFMLIALIGNGLVIWIFST 2 1 AKTLRTPSNIFVVNLAICDFLMMSKTPIFIYNSFKLGYALGHRACQIFALLGSFSGIGASATNAVIAYDRYR 2 1 VIATPFAPKLSRTKAVLYLALVWAYVTPWALLPLFEQWSRFVP 1 2 EGFLTSCTFDYLTPTSEIRNFVTVMFFICYVFPMSLIIYFYSQIVSHVIIHEHNLREQ 0 0 AKKMNVESLRSNANMHTQSAEIRIAKAAITICFLFVASWTPYAVLALIGAYGNQ 2 1 DLLTPAVTMIPACACKAVACVDPYVYAISHPRYR 2 1 QELSKKFPWLDIKEAPAPSSVDANSTATEMTLPTQTSPAEA* 0 >LWS_rhoPro Rhodnius prolixus (kissing_bug) 0 MAQPIGPSFAAYQWGQSANPSANRSVVDMVPPEMLSMVDAHW 2 1 YQFPPLNPLWHGILGFVIGVLGIISIVGNGMVIFIFSSTKTLRTPSNLLVVNLAFSDFLMMFTMSPPMVINCYNETWVL 1 2 GPLMCELYGMLGSLFGCASIWTMTMIALDRYNVIVK 0 0 GISAKPMTNKTAMLRILLVWAFSIMWTVFPFFGWNR 2 1 YVPEGNMTACGTDYLTKNWVSRSYILVYSVFVYFLPLFTIIYSYFFILQ 0 0 AVSAHEKQMREQAKKMNVASLRSAEAANTSAEAKLAK VALMTISLWFMAWTPYLVINYSGIFETISISPLFTIWGSLFAKANAVYNPIVYAIR 2 1 HPKYKQALEKKFPSLSCASPQDDTTSVATGVTTSTDDKAPSA *0 >TMT_rhoPro Rhodnius prolixus (kissing_bug) Insecta; Pterygota ciliary opsin full ACPB01038514 + ACPB01038515 56% TMT_triCas 0 MLMPSAGFLAASIILFLIGFLGFFGNLIVIIIMCRDKN 0 0 LWTPVNFILFNVIVSDFSVAALGNPFTLASAIAKRWFFGQSMCVAYGFFMALL 1 2 GITSINSLTVLALERYLIVSQPVSHGSLSRPTASDIVGSIWLYSFVITiPPLVGWGEYGLEAANIS 2 1 CSINWETRSHSSTSYILFLFTFGFFIPIIVISYSYMNIILTMKK 0 0 STMNAGRVNKAESRVTWMIFVMIFAFFLAWTPYAILALMIAFFDSNVSPAIATIPAIFAKTSICYNPFIYAGLNTQVVYFFV* 0
Hexapoda: Acyrthosiphon pisum (pea_aphid) .. 6 opsins
The first draft of aphid genome Acyr_1.0 was released in June 2008; a surprisingly brief genome paper appeared on 23 Feb 2010 after nine months in review. The contigs are available for tblastn at GenBank in the wgs division. Coding gene annotation was initially low quality, with 11 gene models labeled 'opsins' of which only 6 were valid. However the cryptochrome photosystem received a thorough treatment.
The opsin repertoire of Acyrthosiphon is surprising. First it does not exhibit gene loss (other than peropsin, lost much earlier) because ciliary, long wavelength, blue and ultraviolet opsins are all represented. The latter classes of opsins are expanded into two gene pairs. Contigs are so small that it is not possible to say whether these are tandem. One gene of the four has lost K90 to valine and presumably lacks the associated shift to UV in peak adsorption.
The first pair has 8 exons, the second 3, suggesting (along with lowish percent identity) substantial time since duplication and divergence. The second pair, called UVV2a/b below, has lost the HEK motif of the third cytoplasmic loop, raising issues about retention of Gq as signaling partner.
Five lines of evidence suggest this second pair corresponds to RH7 in Drosophila:
- RH7 are best-blastp match at nr and wgs to aphid query, though percent identity is low at 43%
- large deletion in CL3 causes loss of HEK, though residual residues do not align with CL3 of drosophila
- distinctive match in EL2 of ALDIGLSV region of RH7 to VLDLGYS in aphid including 1 extra residue
- distinctive length and similar motif past DRY motif at boundary of TM4 and CL2
- shares unique 3 exon structure and identical intron location and phases (21 12)
Odd phylogenetic distribution of RH7 within insects: + Insecta Dicondylia Pterygota Neoptera Paraneoptera Hemiptera Acyrthosiphon - Insecta Dicondylia Pterygota Neoptera Paraneoptera Hemiptera Rhodnius + Insecta Dicondylia Pterygota Neoptera Endopterygota Diptera Drosophila - Insecta Dicondylia Pterygota Neoptera Endopterygota Diptera Aedes - Insecta Dicondylia Pterygota Neoptera Endopterygota Hymenoptera Apis - Insecta Dicondylia Pterygota Neoptera Endopterygota Hymenoptera Nasonia - Insecta Dicondylia Pterygota Neoptera Endopterygota Coleoptera Tribolium
>TMT_acyPis Acyrthosiphon pisum (pea_aphid) XM_001952259 wrong ciliary opsin 53% TMT_aedAeg 0 MNGEYAQPQHISDAIYLGAAIVLSIIGIVGFIFNTCVIFIMIRDTR 0 0 LWTPQNVIIFNLATSDLAVSVLGNPVTLAAAITKGWIFGQTICVIYGFFMALF 1 2 GIASITTLTVLAYDRYLMIRYPFSSSRLTKETALYAIAGIWIYAFAVTGPPLFGWNRYVNESANIS 2 1 CSIDWESGEHSNYVIYIFVFGLFLPVTVIIYSYVSLVVTVRK 0 0 AEKIIGQATKAECRVAIMVAVMILAFLTAWMPYSVLALMIAFGGVHISPVVSIIPALCAKSSICWNPIIYIGLNTQ 0 0 FRSAWKRFLNIQDTLSEVSLDADITTGMTKLMTGHQELPAHPMNNGDASHPPGLIMCCLAHDEHRQSATYADRYECNLEMKSCNPQTLGRRPETDIGDVSL* 0 >INSE_LWS_acyPis Acyrthosiphon pisum (pea_aphid) SCAFFOLD6053:23617,25535 67% LWS_pedHum 0 MLNKIGSHYERQENWVAEGGFGNETVVDRVPADMMHLIDPSW 2 1 YQFPPMESMWYKWLGVTIFFLGILSVVGNGMVIYIFTCTKNLRTPSNLLIVNLAFSDFCLMFTMCPAMVWNCFYETWMF 1 2 GPFACELYAMFGSLFGVTSIWTMVFIALDRYNVIVK 0 0 GLSAKPMTTKLALLQIFCIYLHGLFWTLTPFFGWSR 2 1 YVPEANMTACGTDYLTLAWHSRSYVLVYAIFAYYLPLLVIIYAYYFIVK 0 0 AVASHEKSMREQAKKMNVSSLRSGDQSNTSAEFKLAKVALMTISLWFMAWTPYMVINFAGIFQLMTIDPLFTIWGSVFAKANAVYNPIVYAIS 2 1 HPKYRLALDKKFPCLVCGKLEDDRSDSKSVASAQTTISEDKV* 0 >INSE_UVVa_acyPis Acyrthosiphon pisum (pea_aphid) 8 exons SCAFFOLD14509:21417-33525 62% UVV_apiMel V in K90 0 MDFNRSVSRPLSQLGS 2 1 SFMENEEELQLMGWNLTPEDLTHIPEHWLSYPEVRSLYHYILAFSYTILFCLGVIGNGLVLWIFCV 0 0 SKPLRTPSNLFVLNLALCDFSMVLVLPILIYDSIDHKYP GHLQCQIFALCGSISGIGAGATNAAIAYDRYS 2 1 TIAKPFEGRMTYGKALILIICIWIYVLPWCLLPLTEKWNRFVP 1 2 EGFLTSCSFDYLTPTEETKAFVGTMFVICYVIPMSFIIYFYSQIVCHVFNHEKALREQ 0 2 AKKMNVESLRSNQDANAQSAEVRIAKAAITICFLFVAAWTPYAVVAMIGAFGDQ 2 1 SLLTPIASMLPAVFAKTVACFDPYVYAISHPKYR 2 1 LELSKRVPCLGITEKPLATSDTQSITTAA* 0 >INSE_UVVb_acyPis Acyrthosiphon pisum (pea_aphid) 8 exons SCAFFOLD14509:41790,53815 76% identical UVVa_acyPis K in in K90 0 MDFNRTVSRPLAQLGs 2 1 SLMENEVGETHLLGWNLQAEDLIHIPEHWLKYQEPSSLQHYYLAFMYTIFMFVALFGNGLVIWVFCV 0 0 AKPLRTPSNIFVINLALCDFVMMAKAPIFILGSINRGYQ GHFLCQLFGTAGAFSGIGASATNAAIAYDRFS 2 1 TIAKPFDGRMTYGRAFFLIICIWTYTLPWGLLPLTEKWNRYVP 1 2 EGYLTSCTFDYLSPTDETRAFVGIMFVICYVIPVSLVIFFYSQIVSHVFNHEKALREQ0 0 AKKMNVESLRSNQDANAQSAEVRIAKAAITICCLFIASWTPYAVVAMIGAFGDR 2 1 SLLTPGITMIPAIFCKTVACFDPYVYAISHPRYR 2 1 LELSKRVPCLGISEKPPPTASETQSTTTAA* 0 >INSE_UVV2a_acyPis Acyrthosiphon pisum (pea_aphid) 3 exons SCAFFOLD4798:3246-5335 altered HEK CL3 52% UVV2_pedHum K in in K90 0 MIDFKTKYPVNLWKDHGLYTDDYIKLINSHWLKFMPPNPTSHYVLGLLYTVIMVFGCTGNSLVIFMYFK 2 1 CRSLQTPANMLIINLAVSDFIMLAKASVFIYNSYYLGPALGKL 1 2 GCQVCGFLGGLTGTVSIMTLAAISLDRYYVIVCPLKAAVKTTKQRARIWIGLIWIYGFSFSIVPVLDLGYSRYVSEGYLTSCSFDYLSDNDQDKRFI LVFFTAAWCIPFTIILYCYVNILMAVWMTTEIVTSRVGQQEEKRKTDIRLGYMVIGALALWFVSWTPYAVVALLGVFDLKEYISPLSSMIPALFCKAAS CTDPWFYAITHPRFKKELMKLLTKSKSRKLVRNYGMKKGWVGSHLNKNGSVDFDNCLKTEYKEENTTIFMLESDDNNLHCQGSTSGHKTESTKEPETKFTASASQETLKYMLPS* 0 >INSE_UVV2b_acyPis Acyrthosiphon pisum (pea_aphid) SCAFFOLD14504:180756-183351 72% UVV2a_acyPis altered HEK CL3 K in in K90 0 MSDFKTKYPIDTWKEHGFYTDDYMKLINSHWFKFMPPNATSHYILGFLYSVIMVLGCFGNSLVIFMYIK 2 1 CKSLQTPANVLIMNLAVSDFIMLAKTPVFIYNSFYQGPTLGKL 1 2 GCQIYGFFGGLTGTVSIMTLAAISLDRYYVIVHPLNAAVKTTKQRARVWIGLIWIYGFLFSIIPVMDLGYNRYVPEGYLTSCSFDYLSDDNQEKGFILVFFTAAWCIPFTTISYCYIKI LRAVWMTSEMAASRFGQEEEKRKTEIRLGYVVVGVIMLWFVSWTPYAMVALLGVFDRKDYITPLSSMIPAVLCKAASCMDPWIYAITHPRFKNELTKLMSRKKTRKLERDYGMKKNWGGQ SYSNKSGAGLRNLSSSEDECVEEVIVVIDPDDKKMKRQGSTSSHKTEETKALETKFPPTRQESLKYMPPSWYKLPRTTSKSSIMLDPKLTGDDNNK* 0
Hexapoda: Drosophila melanogaster (fruitfly) .. 7 opsins
Every aspect of photoreception in Drosophila has been studied for decades. Because this research is regularly reviewed at length, the focus here is on genome project developments and issues that remain in characterizing opsin function and evolution.
Drosophila has seven opsins, all of melanopsin class. Thus it is far-fetched homologically and anatomically as a model system for vertebrate ciliary vision. Ciliary-class opsins (present spottily elsewhere in arthropods) have been lost in all 12 drosophilid genomes, as have all peropsins (which persist in some chelicerates, lophotrochozoa and deuterostomes). The retinal isomerase regeneration cycle cannot have a counterpart in invertebrates because some species with ciliary opsins lack the peropsins and some species without ciliary opsins retain peropsins.
However the loss of two deeply historic opsin classes in fruitflies raises the question whether ancestral neuroanatomical structures have also been lost. The comparable ciliary opsin in bee is expressed somewhere in the brain but not in simple or compound eyes -- unfortunately it is not known whether anatomical expression is like that of Platynereis nor whether drosophila lacks this structure.
The paired Drosophila retinas have 850 ommatidia each housing eight photoreceptors of three types; the paired cephalopharyngeal Bolwig organs have 12 photoreceptor cells of two types. Oddly, during metamorphosis to eyelet, the outer Bolwig cells die while inner cells switch gene expression from Rh6 to RH5.
Two of the Drosophila opsins have peak sensitivity in the ultraviolet (RH5 RH7) consistent with their K90 lysine and shorter CL3 loop motif, two sister opsins peak (RH3 RH4) in the blue and the rest (RH6,(RH1,RH2)) at longer visible wavelengths. Opsins have been assigned to the four known photoreceptor structures as follows:
- RH5 RH6 Bolwig organ (larva) in founder and peripheral cells, resp.
- RH6 Hofbauer-Buchner eyelet (adult founder cell remnants of Bolwig organ)
- RH2 ocellus (adult)
- RH1 R1-R6 peripheral photoreceptors of ommatidia (adult eye)
- RH3 RH4 R7 photoreceptor of ommatidia (adult eye)
- RH5 RH6 R8 photoreceptor of ommatidia (adult eye)
- RH3 dorsal R7 R8 polarization receptors (adult eye)
Note RH7 is missing from the list. This orphan opsin has no tissue-labeled transcripts at GenBank as of June 2009. It does not occur in any of the known photoreceptors, suggesting the repertoire of adult brain ultrastructures is still incomplete. Some authors have questioned whether RH7 is a 'real' opsin (because the third cytoplasmic loop CL3 is non-standard).
However it still retains the DRY motif, the Schiff base lysine and many other characteristic residues and opsin motifs. Its peak sensitivity would lie in the UV because of the well-conserved K90 motif, which is conserved in all 12 drosophilid genomes. The upstream PAX6 promoter RCSI site still matches the consensus sequence, TAATYCGATTA even though the first coding exon is anomalously lengthened and very prone to internal indels.
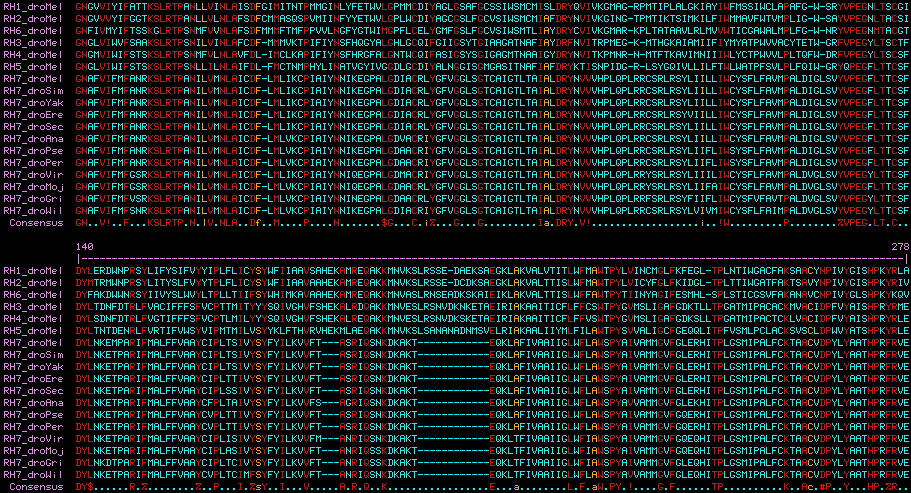
RH7 has three exons versus five in bee UV and eight in bee blue opsins. The first intron in RH7 VIFMYFK 21 CRSLQTP is identical in location and reading phase 21 to an intron in conventional UV opsins. This provides strong independent support to Blast clustering for a shared common ancestry of these opsin classes because a 300 residue protein has 3 possible phases (thus 900 possible introns). This common intron also suggests a tandem or segmental duplication history relating these three genes followed by intron loss, rather than retropositioning followed by intron gain.
The intronation of RH7 within arthropods has been stable back to chelicerates (though the gene itself has been lost in many lineages and Drosophila itself has retained only the second). Astonishingly, Lophotrochozoan melanopsins also have the identical intron pattern of RH7 (determinable from Lottia, Aplysia, Helobdella, Schmidtea, Schistosoma genome projects) as do vertebrate melanopsins (for example Gallus) proving both introns of RH7 ancestral to the Ur-bilateran. None of these latter opsins have ultraviolet K90; indeed some are non-imaging. The only known cnidarian melanopsin, from coral, is a transcript.
RH7 VIFMYFK 21 CRSLQTP Acyrthosiphon UV5 VIWIFCA 21 AKSLRTP Apis UVB VIWIFST 21 SKSLRTP Apis MEL VIYTFSR 21 TKSLRTA Lottia MEL VIYAFCR 21 SRTLQKP Gallus
Other arthropod melanopsins also have unusual cytoplasmic third loops, which has predictive implications for Galpha signaling partner. This Galpha web tool allows studying the effects of replacing cytoplasmic loops or tail of RH7 with those of its nearest match, the UV-tuned RH5. This would not affect transmembrane structure or extracellular loops but might alter coevolved relations on the cytoplasmic face.
RH7 is exceedingly conserved (except in the amino terminus) in the other 11 drosophilids with sequenced genomes, ruling out both processed and unprocessed pseudogenes. Its two introns bear no relation in position and phase to those in any other drosophila opsins. The carboxy terminus is surprisingly conserved despite earlier indels. Remarkably for such a conserved gene, it is quite isolated phylogenetically. Only aphid provides a potential ortholog candidate. This cannot plausibly reflect horizontal gene transfer (from what animal?) but cannot reflect an ancient gene duplication either, short of invoking many lineage-specific gene losses.
Consequently the first order of business is to work up the species tree with targeted sequencing to pinpoint the evolutionary origin of RH7 -- Insecta; Dicondylia; Pterygota; Neoptera; Endopterygota; Diptera; Brachycera; Muscomorpha; Eremoneura; Cyclorrhapha; Schizophora; Acalyptratae; Ephydroidea; Drosophilidae; Drosophila melanogaster group. Because the gene is missing in dipteran and coleopteran genomes (mosquitoes, bee, flour beetle), that search can be be restricted. It seems too diverged from other opsins to have originated just in a few tens of millions of years of evolution represented by drosophilids (but perhaps not too much in terms of generations).
Second, it should be noted that a 2002 whole-proteome quantitative transcription project did in fact uncover RH7 transcripts (as displayed at the UCSC GeneSorter). Here peak expression, as normalized to egg-to-adult total RH7 transcripts, occurred in 76-hour mesomorphs. Total expression was highest in 5-day adult females. Improved all-gene experiments in 2008-09 ruled out RH7 expression in pupae but verified expression in adult male and female heads at equal levels. These transcripts are not yet correlated with any anatomical structure. Despite arrays of the full set of 13,000 coding genes, a Drosophila brain expression atlas has never gotten off the ground -- each gene must be inefficiently studied in a one-off manner.
Recently three RH7 transcripts from a butterfly (Spodoptera littorali, called UV7_spoLit in the reference gene section) became available. Two of these were from male antenna, the other from pooled tissue. Because antenna are easily separated from other tissues, this strongly suggests RH7 is specifically expressed there -- in some anatomical context yet to be determined. Recall the antenna is already a multi-purpose sensory organ: the six segments from proximal to distal are labelled a1-a5 and arista, encoding hygrosensation (arista), thermosensation (a3), olfaction (a3), and audition (arista and a2). The eye and antenna originate in common from the eye-antennal imaginal disc, with Dip3 loss leading to an extra photoreceptor that speculatively might house photoreceptor RH7 when the cell has its usual antennal fate.
Gene, name, coding exons, introns present, chr location: RH1 (CG4550-RA) 5 chr3R 15,712,948 shares two introns with RH6 and one with RH2, similar SKA* termini RH2 (CG16740-RA) 4 chr3R 14,725,942 RH6 (CG5192-RB) 3 chr3R 11,309,650 RH3 (CG10888-RA) 1 chr3R 15,907,472 possible retrogene of RH5 RH4 (CG9668-RA) 2 chr3L 16,850,872 possible retrogene of RH5 with later intercolated genes RH5 (CG5279-RA) 3 chr2L 12,009,111 two ancestral introns (also Apis, Daphnia; first also Aplysia, Platynereis and Homo) RH7 (CG5638-RA) 3 chr3L 12,162,941 two novel introns, anomalous first exon
RH7 appears not involved in Drosophila circadian photoreception systems, which are mediated by the blue sensitive pterin-flavoprotein cryptochrome CRY (not homologous to opsins) in clock neurons and by opsins RH1, RH5 and RH6 in photoreceptors.
Curiously CRY is also implicated in magnetic field perception based anisotropic hyperfine coupling between unpaired electron and nuclear spins ([1, 2, 3). RH7 is not plausibly involved in this either because it is new whereas magnetosensing is old and widespread. In eery analogy to ciliary opsins, drosophilids but not butterflies have lost the close paralog to mammalian CRY.
>RH1_droMel Drosophila melanogaster (fruitfly) CG4550-RA ninaE 0 ME 00 SFAVAAAQLGPHFAPLSNGSVVDKVTPDMAHLISPYWNQFPAMDPIWAKILTAYMIMIGMISWCGNGVVIYIFATTKSLRTPANLLVINLAISDFGIMITNTPMMGINLYFETWVLGPMMCDIYAGLGSAFGCSSIWSMCMISLDRYQVIVKGMAGRPMTIPLALGKIAYIWFMSSIWCLAPAFGWSR 2 1 YVPEGNLTSCGIDYLERDWNPRSYLIFYSIFVYYIPLFLICYSYWFIIA 0 0 AVSAHEKAMREQAKKMNVKSLRSSEDAEKSAEGKLAKVALVTITLWFMAWTPYLVINCMGLFKFEGLTPLNTIWGACFAKSAACYNPIVYGIS 2 1 HPKYRLALKEKCPCCVFGKVDDGKSSDAQSQATASEAESKA* 0 >RH6_droMel Drosophila melanogaster (fruitfly) CG5192-RB gross genomic misassembly exon1 0 MASLHPPSFAYMRDGRNLSLAESVPAEIMHMVDPYWYQWPPLEPMWFGIIGFVIAILGTMSLAGNFIVMYIFTSSKGLRTPSNMFVVNLAFSDFMMMFTMFPPVVLNGFYGTWIMGPFLCELYGMFGSLFGCVSIWSMTLIAYDRYCVIVKGMARKPLTATAAVLRLMVVWTICGAWALM PLFGWNRYVPEGNMTACGTDYFAKDWWNRSYIIVYSLWVYLTPLLTIIFSYWHIMK 0 0 AVAAHEKAMREQAKKMNVASLRNSEADKSKAIEIKLAKVALTTISLWFFAWTPYTIINYAGIFESMHLSPLSTICGSVFAKANAVCNPIVYGLS 2 1 HPKYKQVLREKMPCLACGKDDLTSDSRTQATAEISESQA* 0 >RH2_droMel Drosophila melanogaster (fruitfly) CG16740-RA 0 MERSHLPETPFDLAHSGPRFQAQSSGNGSVLDN 0 0 VLPDMAHLVNPYWSRFAPMDPMMSKILGLFTLAIMIISCCGNGVVVYIFGGTKSLRTPANLLVLNLAFSDFCMMASQSPVMIINFYYETWVLGPLWCDIYAGCGSLFGCVSIWSMCMIAFDRYNVIVKGINGTPMTIKTSIMKILFIWMMA VFWTVMPLIGWSAYVPEGNLTACSIDYMTRMWNPRSYLITYSLFVYYTPLFLICYSYWFIIAAVAAHEKAMREQAKKMNVKSLRSSEDCDKSAEGKLAKVALTTISLWFMAWTPYLVICYFGLFKIDGLTPLTTIWGATFAKTSAVYNPIVYGIS 2 1 HPKYRIVLKEK 0 0 CPMCVFGNTDEPKPDAPASDTETTSEADSKA* 0 >RH3_droMel Drosophila melanogaster (fruitfly) CG10888-RA single exon 0 MESGNVSSSLFGNVSTALRPEARLSAETRLLGWNVPPEELRHIPEHWLTYPEPPESMNYLLGTLYIFFTLMSMLGNGLVIWVFSAAKSLRTPSNILVINLAFCDFMMMVKTPIFIYNSFH QGYALGHLGCQIFGIIGSYTGIAAGATNAFIAYDRFNVITRPMEGKMTHGKAIAMIIFIYMYATPWVVACYTETWGRFVPEGYLTSCTFDYLTDNFDTRLFVACIFFFSFVCPTTMITYY YSQIVGHVFSHEKALRDQAKKMNVESLRSNVDKNKETAEIRIAKAAITICFLFFCSWTPYGVMSLIGAFGDKTLLTPGATMIPACACKMVACIDPFVYAISHPRYRMELQKRCPWLALNEKAPESSAVASTSTTQEPQQTTAA* 0 >RH4_droMel Drosophila melanogaster (fruitfly) CG9668-RA two exons w large intron (no RM but intercolated genes) 0 MEPLCNASEPPLRPEARSSGNGDLQFLGWNVPPDQIQYIPEHWLTQLEPPASMHYMLGVFYIFLFCASTVGNGMVIWIFST SKSLRTPSNMFVLNLAVFDLIMCLKAPIFIYNSFHRGFALGNTWCQIFASIGSYSGIGAGMTNAAIGYDRYNVITKPMNRNMTFTKAVIMNIIIWLYCTPWVVLPLTQFWDRFVP 1 2 EGYLTSCSFDYLSDNFDTRLFVGTIFFFSFVCPTLMILYYYSQIVGHVFSHEKALREQAKKMNVESLRSNVDKSKETAEIRIAKAAITICFLFFVSWTPYGVMSLI GAFGDKSLLTPGATMIPACTCKLVACIDPFVYAISHPRYRLELQKRCPWLGVNEKSGEISSAQSTTTQEQQQTTAA* 0 >RH5_droMel Drosophila melanogaster (fruitfly) CG5279-RA two small introns also seen in Apis, Daphnia; first in Aplysia, Platynereis and Homo 0 MHINGPSGPQAYVNDSLGDGSVFPMGHGYPAEYQHMVHAHWRGFREAPIYYHAGFYIAFIVLMLSSIFGNGLVIWIFST 2 1 SKSLRTPSNLLILNLAIFDLFMCTNMPHYLINATVGYIVGGDLGCDIYALNGGISGMGASITNAFIAFDRYKTISNPIDGRLSYGQIVLLILFTWLWATPFSVLPLFQIWGRYQP 1 2 EGFLTTCSFDYLTNTDENRLFVRTIFVWSYVIPMTMILVSYYKLFTHVRVHEKMLAEQAKKMNVKSLSANANADNMSVELRIAKAALIIYMLFILAWTPYSVVALI GCFGEQQLITPFVSMLPCLACKSVSCLDPWVYATSHPKYRLELERRLPWLGIREKHATSGTSGGQESVASVSGDTLALSVQN* >RH7_droMel Drosophila melanogaster (fruitfly) CG5638-RA long N-terminal has M comp genomics support, EC074058 CO302368, 3 novel exons 0 MEAIIMTTLPNLTTDAGDSSFWLTGALSLSEMLANSSHSHSTGSTTSTAGSSATESSAVNVGKDHDKHVNDSVSTGLS 2 1 NYSNYPSYIHYRDKYDLSYIAKVNPFWLQFEPPKSSTFLIMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLIKCPIAIYNNIKEGPALGDI 1 2 ACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIILLIWCYSFLFAVMPALDIGLSVYVPEGFLTTCSFDYLNKEMPARIFMALFFVAAYCIPLTSIVYSYFYILKVVFTASRIQSNKDKAKTEQ KLAFIVAAIIGLWFLAWSPYAIVAMMGVFGLERHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFYGRGVLRRVSTTRSSYMTRSRSSFTHRLRTSTTGEGGMGDHRMENYLMNNNLMMVPEETEENEEIVVVAEINNSISSVMEQSKF* 0 >RH7_droSim Drosophila simulans (fruitfly) chr3L:11530420 11532815 0 MEAIIMTTLPNLTTDAGDSSFWLTGALSLSEMLANSSHGHSTGSTTSSAGSSATESSAVNVGKDHDKHVNDSVSTGLS 2 1 NYSNYPSYIHYRDKYDLSYIAKVNPFWLQFEPPKSSTFLVMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLIKCPIAIYNNIKEGPALGDI 1 2 ACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIILLIWCYSFLFAVMPALDIGLSVYVPEGFLTTCSFDYLNKETPARIFMALFFVAAYCIPLTSIVYSYFYILKVVFTASRIQSNKDKAKTEQ KLAFIVAAIIGLWFLAWSPYAIVAMMGVFGLERHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFYGRGVLRRVSTTRSSYMTRSRSSFTHRLRTSTTGEGGMGDHRMENYLMNNNLMMVPEETEENEEIVVVAEINNSVSSVMEQSKF* 0 >RH7_droSec Drosophila sechellia (fruitfly) super_0:4344247 4346640 0 MEAIIMTTLPNLTTDAGDSSFWLTGALSLSEMLANSSHGHSTGSTASSAGSSATESSAVNVGKDHGKHVNDSVSTGLS 2 1 NYSNYPSYIHYRDKYDLSYIAKVNPFWLQFEPPKSSTFLVMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLIKCPIAIYNNIKEGPALGDI 1 2 ACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIILLIWCYSFLFAVMPALDIGLSVYVPEGFLTTCSFDYLNKETPARIFMALFFVAAYCIPLSSIVYSYFYILKVVFTASRIQSNKDKAKTEQ KLAFIVAAIIGLWFLAWSPYAIVAMMGVFGLERHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFYGRGVLRRVSTTRSSYMTRSRSSFTHRLRTSTTGEGGICDHRMENYLMNNNLMMVPEETEENEEIVVVAEINNSVSSVMEQSKF* 0 >RH7_droYak Drosophila yakuba (fruitfly) chr3L:12207286 12209654 0 MEAIIMTTLPALTTDAGDSSSFWLTGALSLSEMLANSSHGHSTGSTSSTAGSSATESSTVNVGKDHDVTKHVNDSVSTGLS 2 1 NYSNYPSYIHYRDKYDLSYIAKVNPFWLQFEPPKSSTFLVMAALYFLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLIKCPIAIYNNIKEGPALGDI 1 2 ACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIILLIWCYSFLFAVMPALDIGLSVYVPEGFLTTCSFDYLNKETPARIFMALFFVAAYCIPLTSIVYSYFYILKVVFTASRIQSNKDKAKTEQ KLAFIVAAIIGLWFLAWSPYAIVAMMGVFGLERHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFYGRGVLRRVSTTRSSYMTRSRSSFTHRLRTSTTGDGGMGDHRMENYLMNNNLMMVPEETEENEEIVVVAEINNSVSSVMEQSKF* 0 >RH7_droEre Drosophila erecta (fruitfly) scaffold_4784:12148112 12150459 0 MEAIIMTTLPTLTTDAGDSSFWLTGALSLSEMLANSSHGHSTGSTSSTAGSSATESATVNVGKDHDVAKHVNDSVSTGLS 2 1 NYSNYPSYIHYRDKYDLSYIAKVNPFWLQFEPPKSSTFLVMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLIKCPIAIYNNIKEGPALGDI 1 2 ACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYVIILLIWCYSFLFAVMPALDIGLSVYVPEGFLTTCSFDYLNKETPARIFMALFFVAAYCIPLTTIVYSYFYILKVVFTASRIQSNKDKAKTEQ KLAFIVAAIIGLWFLAWSPYAIVAMMGVFGLERHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFYGRGVLRRVSTTRSSYMTRSRSSFTHRLRTSTTGDGGMGDHRMENYLMNNNLMMVPEETEENEEIVVVAEINNSVSSVMEQSKF* 0 >RH7_droAna Drosophila ananassae (fruitfly) scaffold_13337:1483455 1485125+ frameshifted 0 MEAIILSTLPSLTTNASGSSSHWLTGALSLPEILANSSGSPNTSSADTGSGINLSARDADRHFNISTEAR 2 1 NYSYYPGYIHYRDKYDLSYIAKVNPFWLQFEPPHSSTFLAMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLVKCPIAIYNNIKEGPALGDV 1 2 ACRIYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIILLIWCYSFLFAVMPALDIGLSVYVPEGFLTTCSFDYLNKETPARIFMALFFVAAYCFPLTAIVYSYFYILKVVFSAGRIQSNKDKAKTEQ KLAFIVAAIIGLWFLAWSPYAVVAMMGVFGLEKHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFYGRGILRRVSTTRSSYMTRSRSSFTHPAGRADGGTGRDHRMETYLMNNNLMMVPEETEENEEIVVVAEINNSVSSAIEQSKF* 0 >RH7_droPse Drosophila pseudoobscura (fruitfly) chrXR_group6:2491547 2493151 0 MEALMAALPTLTTEAAGSSLWLTSALSLSEMLANSSTSPNASLVAATTSSAAVATASTTSAAEAVGKVPDKHEVNDNVSTVLS 2 1 TSSSYPGYIHYRDKYDLSYIARVNPFWLQFEPPKSSTFYLMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLVKCPIAIYNNIKEGPALGDA 1 2 ACRIYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIIFLIWSYSFLFAVMPALDIGLSVYVPEGFLTTCSFDYLNKETPARIFMALFFVAAYCVPLTTIVYSYFYILKVVFTASRIQSNKDKAKTEQ KLAFIVAAIIGLWFLAWSPYAIVAMMGVFGQERHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFFGRGVLRRVSTTRSSYMTRSRSSFNRRLRPTPDAEHRVESYLMNNNLMMVPEETEENEEIVVVAEFNNSSYSGMEQSKF* 0 >RH7_droPer Drosophila persimilis (fruitfly) super_9:783822 785423 0 MEALMAALPTLTTEAAGSSLWLTSALSLSEMLANSSTSPNASLVATTSSAAAATASTTSAAEAVGKVPDKHEVNDNVSTVLS 2 1 SYPGYIHYRDKYDLSYIARVNPFWLQFEPPKSSTFYLMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLVKCPIAIYNNIKEGPALGDA 1 2 ACRIYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIIFLIWSYSFLFAVMPALDIGLSVYVPEGFLTTCSFDYLNKETPARIFMALFFVAAYCVPLTTIVYSYFYILKVVFTASRIQSNKDKAKTEQ KLAFIVAAIIGLWFLAWSPYAIVAMMGVFGQERHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFFGRGVLRRVSTTRSSYMTRSRSSFNRRLRPTPDAEHRVESYLMNNNLMMVPEETEENEEIVVVAEFNNSSYSGMEQSKF* 0 >RH7_droWil Drosophila willistoni (fruitfly) scaffold_180949:5140016 5141994+ 0 MDMDMALDMNDAATTTSLWITSAALSLSEILVNTTSHVVTTSPASTSTVETTAVAAVTATGKVVHDDEKHHHHHHHHHQDEVNDNNVTTVLR 2 1 NFSSYPGYIHYRDKYDLSYIAKVNPFWLQFEPPRSSTFYIMAALYCLISVVGCIGNAFVIFMFSNRKSLRTPANILVMNLAICDFLMLVKCPIAIYNNIKEGPALGDI 1 2 ACRIYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLVIVMIWCYSFLFAIMPALDVGLSVYVPEGYLTTCSFDYLNKETPARIFMALFFVAAYCVPLTCIMFSYFYILKVVFTANRIQSNKDKAKTEQ KLTFIVAAIIGLWFLAWSPYAVVAMMGVFGLEQHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLIYGRGVLRRVSTTRSSYITRSRSSFTRRLRTGSELDMRTEPYIMNNNLMMVPEETEENEEIVVVAEINNPSRCVSMHEHTSKF* 0 >RH7_droVir Drosophila virilis (fruitfly) scaffold_13049:6123835 6125790+ 0 METIMSTFPTLTSDDGSLWITSALSEMLTSSSSNSSEAAQNATLVAAAAATTTTVAAAAAAAAANASTAATANVTKVHDKHSHAVNDSETDLR 2 1 CSAYPGYIHYRDKYDLDYIAKVNPFWLQFEPPGTSSFYIMAGLYCLISVVGCFGNAFVIFMFGSRKSLRTPANILVMNLAICDFLMLIKCPIAIYNNIQEGPALGDM 1 2 ACRIYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRYSRLRSYLIIILIWCYSFLFAVMPALDVGLSVYVPEGYLTTCSFDYLNKETPARIFMALFFVAAYCIPLISIVYSYFYILKVVFMANRIQSNKDKAKTEQ KLTFIVAAIIGLWFIAWSPYAIVAMMGVFGQEQHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLMYGRGALRRVSTTRSTYMTRSFTHRMRHTSGDGENRADPYTLNNNLMMVPEETEENDEIIVVAEINNSTSIAMEQSKF* 0 >RH7_droMoj Drosophila mojavensis (fruitfly) scaffold_6680:4445619 4446890+ 0 METIMSTLPTLTADDGSLWITSALTELLASGANSSSGSSSVVADGTQNATFVAAATTTTTTVAAAAAAAAAAAVNASTATTANATKGHHKHPHGVNDSETDLR 2 1 LCSSYPGYIHYRDKYDLTYIAKVNPFWLQFEPPDTSTFYIMAALYCLISVVGCVGNAFVIFMFGSRKSLRTPANILVMNLAICDFLMLVKCPIAIYNNIQEGPALGDA 1 2 ACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRYSRLRSYLIIFAIWCYSFLFAVMPALDVGLSVYVPEGFLTTCSFDYLNKETPARIFMALFFVAAYCIPLASIVYSYFYILKVVFTANRIQSSKDKAKTEQ KLTFIVAAIIGLWFIAWSPYAIVAMMGVFGQEQHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLMYGRGVLRRVSTTRSSYMTRSRSSFTHRLRPSSGDCENRAEPYTLNNNLMMVPEETEENEEIIVVAEINNSISGVMEQSKF* 0 >RH7_droGri Drosophila grimshawi (fruitfly) scaffold_15110:6598464 6600409 0 METIMSTLPTLAADDGSQWLTSALSEVLASSDGRGAAQNATLAAATAVATATTAVNVSKVDDKHLHTVNDSDTDLT 2 1 RCSSYPGYIHYRDKYDLTYIAKVNPFWLQFEPPDTSTFYMMAGLYCLISVVGCFGNAFVIFMFVSRKSLRTPANILVMNLAICDFLMLVKCPIAIYNNINEGPALGDA 1 2 ACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRFSRLRSYFIIFLIWCYSFVFAVTPALDVGLSVYVPEGYLTTCSFDYLNKDTPARIFMALFFVAAYCIPLTCIVYSYFYILKVVFTANRIQSSKDKAKTEQ KLTFIVAAIIGLWFIAWSPYAIVAMMGVFGLEQHITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMIFFGRGVLRRVSTTRSSYMTRSRSSFNHRVRSSSNEGDNRAESYKMNNNLMIVPEETDENEEIIVVAEINNSISIDMEQSKF* 0
Hexapoda: Anopheles gambiae (mosquito) .. 5 opsins
Anopheles is one of several mosquitoes with significant amounts of genomic data available. It is notable for retaining the arthropod ciliary opsin as well as blue, standard UV and RH7 UV ortholog (which in contrast to fellow dipteran Drosophila, has the ancestral intronation). Mosquitoes are often considered to fly primarily under low light conditions (dawn and dusk), conditions under which color vision capabilities might seem largely inoperative. Yet these genes are evolving fairly slowly so evidently under selection so by implication still useful for survival and reproduction.
>UV7_anoGam Anopheles gambiae (mosquito) Diptera XM_308329 0 MGRQGSGNAVRISPSSRNQPYFSSAHLSFVVPFPVHSKYVVRSGYVLPVDPLFVAKINPFWLRFDPPSAGEHYGLAVFYFLMMLFGVIGNALVVFMFYR 2 1 YRSLRTPANYLVINLAVADFIIMMEAPMFIYNSIHQGPALGSI 1 2 GCTVYALMGAVGGTVAIATLTVISIDRYNVVVYPLNPNRSTTKLKCYFLIAFTWAYGLLFASFPALEIGLSRYTAEGYLTACSFDYLDRTYKARVFMFVYFVFAW LIPFAIISYCYARILIAVINANAIQSSKSKNKTEVKLAGVVVGIIGLWFAAWTPYAVVAMMGVFGYEQYLTPLNSMIPAVFAKIAASIDPYFYAMNHPRYRQMLER MFCNRGADQGNSQYQTSHYTRGASRGGDSEGGGGEESGGGGGVGRAPGGGNAGLGRGGTVRGGGGGGRLIAGKGGGGANATGSTGGGGVKALKKQISNGDETSLEVSLEM* 0 >UV5_anoGam Anopheles gambiae (mosquito) Diptera XM_556823 novel short exon 0 MGLVQLDNQTAYRPEALIGADQSGLRYLGWNVPPEELVHIPEHWLQFPEPEASLHYLLGLLYIAFTIFSLVGNGLVIWIFIA 2 1 AKSLRTPSNVFVINLAICDFFMMAKTPIFIYNSFTKGFTLGNLGCQIFGFVGSLT 1 2 GIGAGATNALIAYDR 2 1 YNTITRPFEGRLTQTKAIIFICLIWAYTIPWGVLPLLEIWGRYVP 1 2 EGFLTSCTFDYLSGTFDTRLFVASIFTFSYVLPMSLIIYYYSQIVSHVVNHEKSLREQAKKMNVESLRSNQNQKDASVEIRIAKAAITVC FLFVASWTPYAVLALIGAFGDKSLLTPGVTMFPACACKFVACLDPYVYAISHPRYRIELQKRLPWLAITETLPAENASTCTEQQDGNATTQS* 0 >UVB_anoGam Anopheles gambiae (mosquito) Diptera XM_312478 0 MFLGNESISEGAMLMPMARTAGEMPKLLGWNLPPEEQYLVHDHWKGFPSPPYYMHLMLAMIYFVLMNTSLIGNGIVLWIFGT 2 1 SKSLRNGSNMFIINLAIFDLLMMCEMPMFLVNSFSERLVGYGVGCSVYAALGSMSGIGGAISNAVIAFDRYRTISNPLDGRLSRVQAGLLICLTWLWTMPFTLLPLFEIWGRY IPEGYLTTCSFDYLTDDPDTRVFVGCIFTWAYVIPMIFICYFYARLFGHVRQHEMMLKNQARKMNVESLTANRSEKAQAVEMRIAKAAFTIFFLFVCAWTPYAIVTMIGAFGDR 2 1 TMLTPFVTMVPAVCCKIVSCLDPWVYAISHPKYRQELERRLPWMGIKEADDSVSTTES* 0 >LWS_anoGam Anopheles gambiae (mosquito) Diptera XM_319247 most introns obliterated 0 MPYYGPMQQPGLWGQPVANLTVVDKVPPEIMHLVDPHWSQFPPMNPLWHSIIGFVIFVLGVVSIIGNGMVIYIFSTAKSLRTPSNLFIVNLALSDFLMMGTN AFTMVYNCWFETWSLGLLMCDLYAFFGSLFGCCSIWTMTMIALDRHNVIVHGLSGKPLTNTGAILRILLCWLIGVVWGILPMLGWNRYVPEGNMTACGTDYLTDDWFHKSYILVYS VFVYYTPLFTIIYAYFFIIK 0 0 AVSAHEKNMREQAKRMNVQSLRSSDDGKSTEMKLAKVALVTISLWFMAWTPYTVINYTGVFKTASITPLATIWGSVFAKANAVYNPIVYGISHPKY RAALLRRFPSLACSDGPPADDKSLASEASGITSAGNPTTA* 0 >TMT1_anoGam Anopheles gambiae (mosquito) Gt encephalopsin-class ciliary 461 aa 000 nm no_ref XM_312503 encephalopsin GPROP11 adjacent head-to-head tandem GPROP12 0 MYDVTDAAAINSDHQELMAPWAYNGAAVTLFFIGFFGFFLNIFVIALMYKDVQ 0 0 LWTPMNIILFNLVCSDFSVSIIGNPLTLTSAISHRWLYGKSICVAYGFFMSLL 1 2 GIASITTLTVLSYERFCLISRPFAAQNRSKQGACLAVLFIWSYSFALTSPPLFGWGAYVNEAANIS 2 1 CSVNWESQTANATSYIIFLFIFGLILPLAVIIYSYINIVLEMRK 0 0 NSARVGRVNRAERRVTSMVAVMIVAFMVAWTPYAIFALIEQFGPPELIGPGLAVLPALVAKSSICYNPIIYVGMNTQ FRAAFWRIRRSNGVAGQPDSNNTNNSNRDKESARHTAKEGL ECSLDFCHWTVRGTRVSISSAERNVPAPAARERSGGHSVTGSREESRDRHVTLKTMLSVGPRSPSSVAPVAADCSTTDVPTSGDGSVRIVRQDSELSVIHDGGGGGGGSSSRVLVIKSQKPRSNML* 0
Hexapoda: Bombyx mori (moth) .. 5 opsins
The Dec 2008 silkworm genome contains the full set of opsins expected from its phylogenetic position. Coverage of the assembly is excellent but multiplicity issues exist for assembly contigs. LMS in particular has a closely following tandem pair that may be an assembly artifact, heterozygosity error, partial segmental pseudogene, or recent functional duplication. The protein sequences are very close to Manduca (which however lacks a ciliary opsin). The full set of opsins were not available to authors reconstructing the ancestral lepidopteran eye and have no entries at GenBank despite a dedicated silkworm genomic site and alternative blast site.
Silkworm opsins were experimentally studied in a brief but interesting 2001 BBRC paper. Larval brain (but not sub-esophageal or thoracic ganglia) expressed 'boceropsin' bilaterally in defined cells suggesting but not establishing a role in photoperiodism. This gene is reportedly regulated by microRNA bmo-miR-92.
LMS_bomMor Bombyx mori (moth) Ecdy.Inse.Lepi BAAB01068714 11549248 full G? Bcop boceropsin Bomopsin1 tandem misassembly hemocyte fat body TMT_bomMor Bombyx mori (moth) Ecdy.Inse.Lepi BY930435 frag G? ovary cDNA UV5_bomMor Bombyx mori (moth) Ecdy.Inse.Lepi BABH01012019 frag G? UV7_bomMor BGIBMGA012539-TA|BGIBMGA012539-PA|IPR000276|Rhodopsin-like GPCR superfamily, IPR001760|Opsin, IPR000856|Opsin RH3/RH4 UVB_bomMor Bombyx mori (moth) Ecdy.Inse.Lepi BABH01016294 full
Hexapoda: Apis mellifera (bee) .. 5 opsins
The bee genome has proven quite instructive in terms of ancestral information, in terms of both gene retention and conservation of intron patterns.
It's worth noting that the two long wavelength genes are tail-to-tail --><-- tandems on the same contig, AADG05005335. This arrangement is also seen in wasp and likely ant implying it is ancestral to Hymenoides. The two genes differ by some fusions in their intronation and this too is moderately deep ancestrally.
The transcript situation is still poor however. Apis has five opsins, including a ciliary (pteropsin) opsin but lacks an RH7 ortholog. The ciliary opsin was localized to head but never pinpointed anatomically, prohibiting comparisons to Platynereis.
The bumblebee genome (Bombus terrestris) became available in Feb 2010 in the form of blastn-accessible SRA 454 reads. Coverage seems both uniform and quite high, suggesting its complete opsin repertoire could be recovered. It has the expected ciliary opsin but no UV7 or peropsin.
>UV5_apiMel Apis mellifera (bee) AF004169 353 nm 5 exons Arthropoda Insecta complete genNow 0 MSNDSIHWEARYLPAGPPRLLGWNVPAEELIHIPEHWLVYPEPNPSLHYLLALLYILFTFLALLGNGLVIWIFCA 2 1 AKSLRTPSNMFVVNLAICDFFMMIKTPIFIYNSFNTGFALGNLGCQIFAVIGSLTGIGAAITNAAIAYDRYS 2 1 TIARPLDGKLSRGQVILFIVLIWTYTIPWALMPVMGVWGRFVPEGFLTSCSFDYLTDTNEIRIFVATIFTFSYCIPMILIIYYYSQIVSHVVNHEKALREQAKKMNVDSLRSNANTSSQSAEIRIAK 0 0 AAITICFLYVLSWTPYGVMSMIGAFGNKALLTPGVTMIPACTCKAVACLDPYVYAISHPKYR 2 1 LELQKRLPWLELQEKPISDSTSTTTETVNTPPASS* 0 >UVB_apiMel Apis mellifera AF004168 439 nm 8 exons Arthropoda Insecta complete genNow 0 MLLHNKTLAGKALAFIAEEG 2 1 YVPSMREKFLGWNVPPEYSDLVHPHWRAFPAPGKHFHIGLAIIYSMLLIMSLVGNCCVIWIFST 2 1 SKSLRTPSNMFIVSLAIFDIIMAFEMPMLVISSFMERMIGWEIGCDVYSVFGSISGMGQAMTNAAIAFDRYR 2 1 TISCPIDGRLNSKQAAVIIAFTWFWVTPFTVLPLLKVWGRYTT 1 2 EGFLTTCSFDFLTDDEDTKVFVTCIFIWAYVIPLIFIILFYSRLLSSIRNHEKMLREQ 0 0 AKKMNVKSLVSNQDKERSAEVRIAKVAFTIFFLFLLAWTPYATVALIGVYGNR 2 1 ELLTPVSTMLPAVFAKTVSCIDPWIYAINHPR 2 1 YRQELQKRCKWMGIHEPETTSDATSAQTEKIKTDE* 0 >LWSa_apiMel Apis mellifera (bee) Gq 386 aa 16291092 NM_001077825 rhabdomeric AmLop2 long wavelength ocelli not compound 0 MDTLNITTSFFIEVMPSNISTLTTTGPQFARQLMRFNNQTVVSKVPEEMLHLIDLYW 2 1 YQFPPLDPLWHKILGLVMIILGIMGWCGNGVVVYVFIMTPSLRTPSNLLVVNLAFSDFIMMGFMCPPMVICCFYETW 0 0 VLGSLMCDIYAMVGSLCGCASIWTMTAIALDRYNVIVK 0 0 GMSGTPLTIKRAMLQILGIWLFGLIWTILPLVGWNR 2 1 YVPEGNMTACGTDYLSQDWTFKSYILVYSFFVYYTPLFTIIYSYYFIVS 0 0 AVAAHEKAMKEQAKKMNVTSLRSGDNQNTSAEAKLAK 0 0 VALTTISLWFMAWTPYLVINYIGIFNRSLITPLFTIWGSLFAKANAIYNPIVYGIS 2 1 HPKYRAALKEKLPFLVCGSTEDQTAATAGDKASEN* 0 >LWSb_apiMel Apis mellifera U26026 529 5 exons Arthropoda Insecta 540 complete genNow 0 MIAVSGPSYEAFSYGGQARFNNQTVVDKVPPDMLHLIDANWYQYPPLNPMWHGILGFVIGMLGFVSVMGNGMVVYIFLSTKSLRTPSNLFVINLAISDFLMMFCMSPPM 0 0 VINCYYETWVLGPLFCQIYAMLGSLFGCGSIWTMTMIAFDRYNVIVKGLSGKPLSINGALIRIIAIWLFSLGWTIAPMFGWNR 2 1 YVPEGNMTACGTDYFNRGLLSASYLVCYGIWVYFVPLFLIIYSYWFIIQAVAAHEKNMREQAKKMNVASLRSSENQNTSAECKLAK 0 0 VALMTISLWFMAWTPYLVINFSGIFNLVKISPLFTIWGSLFAKANAVYNPIVYGIS 2 1 HPKYRAALFAKFPSLACAAEPSSDAVSTTSGTTTVTDNEKSNA* 0 >TMT_apiMel Apis mellifera (bee) Gt ciliary 329 aa 16291092 NM_001039968 ciliary AmLop2 compound eye not ocelli pteropsin clock 0 MSLNRSTMEHVIYEDQVSPVMYIGAAIALGFIGFFGFTANLLVAIVIVKDAQILWTPVNVILFNLV 0 0 FGDFLVSIFGNPVAMVSAATGGWYWGYKMCLW 2 1 YAWFMSTLGFASIGNLTVMAVERWLLVARPMQALSIR 2 1 HAVILASFVWIYALSLSLPPLFGWGSYGPEAGNVSCSVSWEVHDPVTNSDTYIGFLFVLGLIVPVFTIVSSYAAIVLTLKKVRKRA 1 2 GASGRREAKITKMVALMITAFLLAWSPYAALAIAAQYFN 0 0 AKPSATVAVLPALLAKSSICYNPIIYAGLNNQFSRFLKKIFDARGSRTAVPDSQHTALTALNRQEQRK* 0 >TMT_bomTer Bombus terrestris (bumblebee) Ecdy.Inse.Hyme 454SRA -------- full G? --- pteropsin presumed ciliary compound eye not ocelli clock 0 MSLNRSTVDNVVYDAEDQVSPMVYVAAAIALGFIGFFGFTMNLLVAIVIVKDAQILWTPVNVILVNLV 0 0 VGDFLVAAFGNPVAMISAITGGWYWSYEMCLW 2 1 YAWFMSTLGFASIGNLTVMAVERWLLVARPMKALSIR 2 1 HAITLGIFVWFYALCLSLPPLFGWGSYGPEAGNVSCSVSWEVHDPLTKSDSYIAFLFVFGLIIPVLVISSSYVAIIVTLRKVRKRA 1 2 GASGRREAKITKMVALMITAFLLAWSPYAALAIAAQYFD 0 0 AKPSASVAVLPALLAKSSICYNPIIYAGLNSQFPRSLKKIFDVRIARSSLPDSQNTALTLLNRQEQRN* 0
Hexapoda: Atta cephalotes (ant) .. 5 opsins
A high-coverage genome assembly of Costa Rican leafcutter ant appeared at NCBI WGS in June 2010. Although contigs are fairly small in an absolute sense, they are large enough relative to opsin gene sizes for complete gene sequences to be recovered (and even some close-in synteny).
This ant genome has the expected complement of five opsins, each of which is closest in percent identity to its apparent ortholog in bee genome. (Both species are hymenoptera.) Nothing is known about sites of expression; an head photograph shows compound eyes. While the two pairs of long wavelength and ultraviolet opsins are likely expressed there, the sole ciliary opsin may be not be involved in imaging vision.
As with bee, no peropsin or counterpart to drosophila UV7 can be found in this species of ant. This suggests loss of the latter in stem Hymenoptera and loss of the former much earlier as Mandibulata separated from Chelicerata. Since the function of these opsins when retained is not known for any arthropod, it is not possible to understand the biological significance of their loss.
Note intracellular helix 8 of ant ciliary TMT opsin ends anomalously in FP rather than the expected FR (or less commonly FK), recalling similar anomalies FP in honeybee and FS in bee (but not in other arthropod orthologs, ruling out sequence error). A possibly coupled development is the alteration of the ERY motif to ERF or ERW in hymenopterids. These motifs are ultra-conserved within GPCR and directly involved in the ionic lock and signal transduction, so the loosening of the constraints within Hymenoptera suggests an altered mechanism of action for ciliary opsins here.
Ant opsins are intronated about as expected but with several gains or losses relative to bee. These have some value in working out ancestral intronation patterns, though describing every instance of intron churning in Insecta is a low priority.
TMT_attCep ERF KSSICYNPIIYASMTAQFP TMT_apiMel ERW KSSICYNPIIYAGLNNQFS TMT_bomTer ERW KSSICYNPIIYAGLNSQFP LMSa_attCep DRY KANAIYNPIVYGIRHPKYR LMSb_attCep DRY KANAVYNPIVYGIRHPKYR UV5_attCep DRY KFVACLDPYVYAISHPRYR UVB_attCep DRY KTVSCIDPWIYAINHPRYR >TMT_attCep Atta cephalotes (ant) Ecdy.Inse.Hyme full wgs ADTU01001608 63% TMT_apiMel 0 MSLNLSVEEQLSIDIYIFAAIALGFIGFL 2 1 GFFLNLLVIITVLKNANVLWTPNNVVLINMV 0 0 IGDFLVAALGNPFTMTSAIAGEWFWSHEVCLW 2 1 YAWFMTTMGFASIGNLTVMAMERFLLVTCPMKTLSIR 2 1 HAYILAILVWMYALSLSLPPFFNWGIYGPEAGNISCSVSWEIHDPYMHNDTYIGFLFIVGFFLPVTIIISSYYGIIKTLRKIRKRV 1 2 GAKNRREKKVTKMVYLMILAFLIAWSPYAVLALATQYFYV 0 0 QTSHILAVLPALLAKSSICYNPIIYASMTAQFPTWKKMFSINRNNTKSKQRHGSELQIK* 0 >LMSa_attCep Atta cephalotes (ant) Ecdy.Inse.Hyme wgs ADTU01020830 16601190 full no fused distal exon iMet uncertain LMSa_apiMel 80% 0 MAFADVTRTIGPRAMQQIMSFNNKTVVNKVPPDMMHLIDPHW 2 1 YQFPPMNPMWHKILGLVMIVLGFMGWCGNGVVVYIFLMTPSLRTPSNLLIVNLAFSDFIMMIIMTPHMMINCYYETWIL 1 2 GPLMCDMHAMVGSLCGCASIWTMTAIALDRYNVIVK 0 0 GMAGKPLTIKKALLEILIIWLFASIWAILPIVGWNR 2 1 YVPEGNMTACGTDYLTKDWSSKSYILVYSIFVYYMPLLIIIYSYYFIVS 0 0 TVAAHERTMREQAKKMNVQSLRSGDNQNISAEAKLAK 0 0 VALMTISLWFMAWTPYLVINYVGIFGIAKISPLFTIWGSLFAKANAIYNPIVYGIR 2 1 HPKYRAALKEKLPFFVCGSTEDPSVAAKTAETSSTTTT* 0 >LMSb_attCep Atta cephalotes (ant) Ecdy.Inse.Hyme full wgs ADTU01020829 18362345 LMSb_apiMel 78% 0 MSFVSGPSHAAYTWAAQGGGFGNQTVVDKVPPEMLHMVDVHWYQFPPMNPLWHGLLGFAIGVLGTISIIGNGMVIYIFTTTKSLRTPSNLLVVNLAISDFLMMLWMSPAM 0 0 VINCYYETWVLGPLFCELYGMMGSLFGCGSIWTMTMIAFDRYNVIVKGLSAKPMTIKGALIRIFAIWLFTILWTIAPLFGWNR 2 1 YVPEGNMTACGTDYLTKDIVSRSYILFYSIFVYFMPLFLIIYSYFFIIQAVAAHEKNMREQAKKMNVASLRSAENQSTSAECKLAK 0 0 VALMTISLWFMAWTPYLIINFAGIFETTKISPLFTIWGSLFAKANAVYNPIVYGIR 2 1 HPKYRAALFQRFPSLACATEPAPGADTMSTTTTVTEGEKSVA* 0 >UV5_attCep Atta cephalotes (ant) Ecdy.Inse.Hyme full wgs ADTU01002850 83% UV5_apiMel 0 MVNESIHWEARVLSAEPIRLLGWNVPAEELIHIPEHWLIYPEPNPSLHYLLAIVYIIFTFVALLGNGLVIWIFCS 2 1 AKSLRTSSNLFVVNLAFCDFFMMIKAPIFIYNSFNTGFATGHLGCQIFAFIGSLSGIGAGMTNAAIAYDRYS 2 1 VIARPLDGKLSRGQVILFIVLIWAYTIPWALMPIMHVWGRFVPEGFLTSCTFDYLTDTPEIQYFVATIFTFSYCIPMSLIIYYYSQIVNHVIDHEKALREQAKKMNVESLRSNVSANTQTMEIRIAK 0 0 AAITVCFLFVLSWTPYGVLAMIGAFGDKTLLTPGITMIPACTCKFVACLDPYVYAISHPRYR 2 1 LELQKRLPWLELQEKSTDTQSSTSEVVNVPSS* 0 >UVB_attCep Atta cephalotes (ant) Ecdy.Inse.Hyme wgs ADTU01025579 ADTU01025580 frag 74% bee 0 2 1 DVQPLQEEHFLGWNFPPEHADLVHPHWRVFLAPGRLWHVALALIYFLLLMLSLLGNGIVIWIFST 2 1 SKSLRTPSNIFILNLAVFDLLMATEMPMLIINSFLERTIGWETGCDVYALLGAISGMGQAITNAAIAFDRYR 2 1 TISCPIDGRLNGKQAMVLALFTWFWALPFSILPMTGLWGRYVA 1 2 EGFLTTCSFDYLTDDEDTRTFVITIFFWAYVLPMLLIIYFYSQLLKSIRSHEKMLRDQ 0 0 AKKMNVKSLVSNQNKERSVEMRIAKVAFTIFFLFVLAWTPYAVVALIGAYGNR 2 1 EALTPFSTMLPAIFAKTVSCIDPWIYAINHPR 2 1 YRQELEKRCKWMGIREKVAEDTVSAQTEKVNAEET* 0
Hexapoda: Nasonia vitripennis (jewel_wasp) .. 4 opsins
The jewel wasp genome contains 4 opsins: one each for UV and blue and a facing tandem pair --><-- with 1 kbp separation for long wavelength. No RH7-type UV nor ciliary opsin is present at the current level of coverage, even though the later is present in another Hymenopteran, the bee.
The two LWS paralogs are intronated somewhat differently. Using outgroups, it can be seen that 4 events (two intron losses and two gains) are needed to synchronize intron patterns. None of these events happened in Nasonia because they also occur in Apis. Two others go back at least to the common ancestor with chelicerates.
Nasonia giraulti and Nasonia longicornis also have 2010 genomic data accessible by tblastn at GenBank wgs. However these species are too close phylogenetically to Nasonia vitripennis for their orthologous opsins to be informative, having diverged in the last million years. When cured of the intracellular bacteria Wolbachia interspecies hybrid readily occur. Unsurprisingly their UV5, UVB and LMS sequences are basically identical. They too lack UV7, ciliary opsins and peropsins. Consequently there is no comparative genomics interest to these genomes except possibly for determining or extending opsin syntenies.
However the phylogenetic tree provided in this article helpfully clarifies both phylogenetic relationships among insects with implications for interpreting their opsins and the immense divergence dates involved. Clearly both crustacean and chelicerate opsins need to be much more deeply sampled given these distant relationships. The time scale provided shows insects diversifying far earlier than angiosperm plants and places the divergence with chelicerates 60 million years earlier than the Cambrian.
The derivation of these dates is explained on page 33 of the 165 page supplemental online section:
"Dating of the split between Nasonia and Apis: Despite continuing uncertainty
about relationships among major hymenopteran lineages (ref 68 below), a reasonable time estimate for the divergence between Nasonia and Apis would be at least 160 MY, probably closer to 190 MY. We therefore use an estimate of 180MY in
this figure.
Fossils assignable to Aculeata (which contains Apis) and
Chalcidoidea (which contains Nasonia) are known back to 130-140 MY (69), so each lineage must be at least that old. Other distinct parasitoid lineages also date back this far based on both fossil and molecular divergence time evidence (70, 71). Moreover, likely relatives of the Chalcidoidea, the Proctotrupoidea, are known from fossils about 160-180 MY old, and some questionable aculeate fossils are known back to about 150 MY (69). Hymenopteran fossils in the
broad sense are known back to more than 200 (maybe as old as 230) MY.
Dating of the split between Nasonia and other Holometabola: Given the recent
finding from genomic comparisons that Hymenoptera are the earliest-diverging among the extant holometabolous insect orders (72, 73) the divergence times between Nasonia and each of Tribolium, Bombyx, Anopheles and Drosophila should be the same, estimated at roughly 290-300 MY. A popular alternative view, that Hymenoptera are more closely related to the panorpoid complex of orders rather than more basal in divergence than Coleoptera, is also supported by some evidence and if accepted, would imply that Nasonia and Bombyx, Anopheles and Drosophila share a common ancestor more recently (roughly 260 MY) than does Tribolium (69). But current evidence, including a recent study based on analysis of a large number of nuclear genes (74), more strongly supports the Hymenoptera-basal position (68). In addition, this recent study suggests that the Hymenoptera/other Holometabola split may have occurred even earlier, as much as 350 MYA (74), although that timing is not supported
by fossil evidence at this point.
Dating of the split between Nasonia and Exopterygota: The split between
Holometabola and the Paraneopteran orders, including Rhodnius, Acyrthosiphon and Pediculus, has been estimated at roughly 300 MY (69), or just slightly older than the oldest divergences within Holometabola. Permian fossil deposits display an astonishing array of insect orders, only some of which survived the end- Permian extinction just under 250 MY. Current evidence suggests that much of winged insect ordinal diversity arose between 330 and 300 MY, a relatively short period of time compared to the long time since (69). It is thus not surprising that relationships among many insect orders have been difficult to resolve even with significant effort from both comparative morphologists and molecular
systematists (75).
Dating of the split between Nasonia and Daphnia: After decades of uncertainty
and dispute concerning higher arthropod relationships, current evidence seems to be definitively resolving the sister-group of Hexapoda to be among the Crustacea (76). The origin of Pancrustacea has been estimated at about 600 MY based on molecular evidence (76), while clear fossil evidence of Arthropoda does not appear until 60 MY later. In any case, the common ancestor between Daphnia and Nasonia would be more recent than this (probably close to but under 500 MY), since Daphnia belongs to one of the crustacean clades close to
Hexapoda (77).
Dating of the split between Nasonia and Ixodes: It is currently uncertain how
old the split between Hexapoda and Chelicerata (including Ixodes) is, but it is clearly early Cambrian to Precambrian in age, or in the vicinity of 600 MY (76, 77). Relationships of Chelicerata to other possibly basal arthropod lineages are
still being explored (76, 78)"
68. M. J. Sharkey, Zootaxa 1668, 521 (2007). 69. D. Grimaldi, M. S. Engel, Evolution of the Insects.Cambridge University Press, Cambridge, U.K., 2005 70. D. L. J. Quicke, H. H. Basibuyuk, M. G. Fitton, A. P. Rasnitsyn, Zoologica Scripta 28, 175 (1999) 71. J. B. Whitfield, Proc. Natl. Acad. Sci. U.S.A. 99, 7508 May 28, 2002 72. J. Savard et al., Genome Res. 16, 1334 Nov 2006 73. V. Krauss et al., Mol. Biol. Evol. 25, 821 May 2008 74. B. M. Wiegmann et al., BMC Biol 7, 34 2009 75. J. B. Whitfield, K. M. Kjer, Annu. Rev. Entomol. 53, 449 2008 76. J. C. Regier et al., Syst. Biol. 57, 920 2008 77. J. C. Regier, J. W. Shultz, R. E. Kambic, Proc. R. Soc. London Ser. B , 395 Feb 22, 2005 78. S. J. Bourlat, C. Nielsen, A. D. Economou, M. J. Telford, Mol. Phylogenet. Evol. 49, 23 Oct 2008 >UV5_nasVit Nasonia vitripennis (jewel_wasp) XM_001608024 wrong, transcripts GE436449 GE390962, very similar Apis 0 MPYYNWNGTDQTAGWPEARIQPAGAPRLLGWNVPPEELVHIPEHWLVYPEPNPALHYLLALLYILFTFVALLGNGLVIWIFCA 2 1 AKSLRTPSNMFVVNLAICDFMMMLKTPIFIYNSFHTGFALGNLGCQIFSFIGSLSGIGASITNAAIAYDRYS 2 1 TIARPLDGKLSRGQVMMLIVLIWMYTIPWALMPSMGVWGRFVP EGFLTSCTFDYITDSDEIRYFVGTIFTFSYAIPMTLIIYFYSQIVGHVVNHEKALREQAKKMNVESLRSGQNKDQASAEVRIAK 0 0 VALTICFLFVAAWTPYGVMSLIGAFGNK SLLTPGVTMIPACCCKAVACLDPYVYAISHPRYR 2 1 LELQKRMPWLELQEKPPASDATSTTTEAVPASS* 0 >UVB_nasVit Nasonia vitripennis (jewel_wasp) XM_001604572 ES636068 0 MAFVGLNGAMGGMGPA 1 2 EKPLQRYSQGPQMQEHLLGWNHPPEHIDIVHPHWRGFLAPGKYWHIGLALIYFMLLVLSFVGNGCVVWIFST 2 1 SKVLRTPSNLFIINLALFDLVMALEIPMLIINSFIERMIGWGLGCDIYAALGSVSGIGSAITNAAIAYDRYR 2 1 TISCPIDGRLNGKQAAVMVAFTWFWTMPFTILPFAKIWGRYTT 1 2 EGFLTTCSFDFLSDDQDTKVFVAAIFSWSYCFPMVLIIYFYSQLIKSVRRHEKMLREQ 0 0 QAKKMNVKSLSAQDKERSVEMRIAKVAFTIFFLFVCSWTPYAVVTMIAAFGNR 2 1 ELVTPFSSMLPAVFAKTVSCIDPWVYAINHPR 2 1 YRQELTKRCQWMGIHEPDSGPSQNNAEAVSVTTEKLKSDDA* 0
>LWSa_nasVit Nasonia vitripennis (jewel_wasp) XM_001606013 GE417061 22063-23541 - strand of AAZX01007316 -->1 kbp <-- 0 MGPSFLTLTAMAQRGGYGGGGGFGGGFNNQTVVDKAPPEIHHMIDPYWYQFPPMNPLWYGILGFVIGCLGCISVAGNGMVVYIFASTKSLRTPSNLLVINLAFSDFCMMFTMSPPM 0 0 VINCYYETWVFGPLMCEIYALCGSIFGCGSIWTMCMIAFDRYNVIVKGLSAKPMTINGSLLRILGIWLMASIWTIAPMFGWNR 2 1 YVPEGNLTACGTDYFSKDWVSRSYIVVYSFFVYFLPLFMIIYSYYFIIKAVSAHEKNMREQAKKMNVASLRQGDSQSAENKLAK 0 0 IALMTISLWFMAWTPYLVINWAGIFDLARLTPLFTIWGSVFAKANAVYNPIVYGIS 2 1 HPKYRAALFARFPSLACAGDAPAGAASDAVSTTSGVTTLTDHDKSNA* 0 >LWSb_nasVit Nasonia vitripennis (jewel_wasp) tandem pair to LWSa, fairly diverged 19237-21046 + strand of AAZX01007316 0 MEHPIVAAGVNATGEFDASSGSASSTTTMVTTAAVQVASTIGPHFARQVMRGFGNLTVVDKVPPEMLHLVGPHW 2 1 YQFPPLWPIWHKLLGVVMIFIGVLGWCGNGMVVYIFLVTPSLRTPSNLLVINLAFSDFVMMIIMSPPMVVNCWYETW 0 0 ILGPLMCDIYALIGSLCGGASIWTMTAIAYDRYNVIVK 0 0 GMSGTPLTIPRALVQIVLIWTHGLIWAMLPLFGWNR 2 1 YVPEGNMTSCGTDYVSDDWLGKSYILVYSIFVYYTPLFSIILCYWHIVS 0 0 AVAAHERGMREQAKKMNVASLRSGDQSGESAEVKLAK 0 0 VAVTTISLWFLAWTPYLVTNYMGIFAKQHVSPLFTIWASLFAKTNACYNPIVYGIS 2 1 HPKYRAGLKVKCPCLVFGDTEDKPKPAAATPAADAASTHSKA* 0
Arthropod opsin gene tree .. 79 opsins
Unalignable N- and C-terminal residues are trimmed off below. The gene tree below arises from their alignment. Note that lophotrochozoan and deuterostome melanopsins cluster together to the exclusion of arthropod genes. The latter fall into two primary clusters of UV and long wavelength. The Rh7 group of UV opsins diverges fairly early within the gene tree. The sole cnidarian gene in this class does not quite form an outgroup but instead nests within ecdysozoan melanopsins. Various outliers in Branchiopoda might indicate the beginning of new sub-clades but the more basal Chelicerates need far better representation.
The nomenclature used here seeks to convey both gene classification and peak wavelength in a few letters that additionally avoid conflict with deuterostome gene names and bow somewhat to Drosophila opsin numbering (where all ecdysozoan genetic work takes place). Thus UV7 and UV5 consist of ultraviolet-peaking opsins closely related to Drosophila Rh7 and Rh5, respectively. If the lysine determinant at position 90 is a blue-shifting residue instead, that is denoted by UVB. Such substitutions may have occurred in both directions multiple times. Similarly long and middle wavelength sensitivity is denoted as LMS. The BCR series derives from founder sequences BcRh1 in the crab Hemigrapsus. The fasta header of the reference sequences contains various literature and site synonyms. When in doubt, a simple text search of 4-5 residues will resolve nomenclature uncertainty.
Species with opsin data (taxa taken from GenBank taxonomy). Note many important groups (eg myriapods and onychophorans) have no opsin data. Insecta Pterygota Neoptera Paraneoptera Hemiptera Acyrthosiphon Insecta Pterygota Neoptera Paraneoptera Hemiptera Rhodnius Insecta Pterygota Neoptera Paraneoptera Hemiptera Homalodisca Insecta Pterygota Neoptera Paraneoptera Hemiptera Megoura Insecta Pterygota Neoptera Paraneoptera Phthiraptera Pediculus Insecta Pterygota Neoptera Endopterygota Diptera Drosophila Insecta Pterygota Neoptera Endopterygota Diptera Anopheles Insecta Pterygota Neoptera Endopterygota Hymenoptera Apis Insecta Pterygota Neoptera Endopterygota Hymenoptera Nasonia Insecta Pterygota Neoptera Endopterygota Coleoptera Tribolium Insecta Pterygota Neoptera Endopterygota Coleoptera Luciola Insecta Pterygota Neoptera Endopterygota Lepidoptera Manduca Insecta Pterygota Neoptera Endopterygota Lepidoptera Papilio Insecta Pterygota Neoptera Orthopteroidea Orthoptera Schistocerca Insecta Pterygota Neoptera Orthopteroidea Orthoptera Dianemobius Crustacea Branchiopoda Phyllopoda Diplostraca Daphnia Crustacea Branchiopoda Phyllopoda Notostraca Triops Crustacea Branchiopoda Sarsostraca Anostraca Branchinella Crustacea Malacostraca Eumalacostraca Eucarida Hemigrapsus Crustacea Malacostraca Eumalacostraca Eucarida Portunus Crustacea Malacostraca Eumalacostraca Hoplocarida Neogonodactylus Chelicerata Merostomata Xiphosura Limulus Chelicerata Arachnida Acari Ixodes Chelicerata Arachnida Araneae Plexippus Chelicerata Arachnida Araneae Hasarius
In the alignment, red indicates residues conserved in almost all opsins and even GPCR, blue residues less conserved but sometimes indicative of opsin class, indels are often diagnostic.
Landmarks are marked up including ultraviolet K90, DRY, Schiff K, NPxxYxxxxxFR, transmembrane regions, informative indels, phyloSNPs etc. Intron boundaries are also be informative.
Special res ..........................................................................UV.............................................DRY...........................................
UV7 diagnos ....................................................................................................................................++..............................G..
UV diagnos ..........................................................................-......................................................P.................................+...
Location ETETETETETETETETETETETM1M1M1M1M1M1M1M1M1M1M1M1MC1C1C1C1C1C1M2M2M2M2M2M2M2M2M2M2ME1E1E1E1E1E1E1E1E1E1E1E1EM3M3M3M3M3M3M3M3C2C2C2C2C2C2C2C2C2C2M4M4M4M4M4M4M4M4M4M4ME2E2E
UV7_aedAeg EDAFRDRINPFWLQFDPPSRTAHYILGFIYFMMMMFGLCGNLLVILMFFRFKSLRTPANYLVINLAIADFIIML-EAPLFVYNSY--HQGPATGNVWCTIYALLGAVGGTVAIVTLTMISIDRYNVVVYPLNPKRSTTRLKVALMIVFAWIYGLVFSVIPALDIGLS
UV7_culQui EDAFRDRINPFWLQFEPPSPVAHYALGFVYFLMMVWGLFGNVLVIFMFFKFKSLRTPANYLVINLAVADFLIML-EAPIFVYNSY--HLGPAFGNTLCTIYSLLGAIGGTVAIMTLTMISVDRYNVVVYPLNPNRSTTRLKVMLMIVFTWIYALVFSLMPALEIGLS
UV7_anoGam DPLFVAKINPFWLRFDPPSAGEHYGLAVFYFLMMLFGVIGNALVVFMFYRYRSLRTPANYLVINLAVADFIIMM-EAPMFIYNSI--HQGPALGSIGCTVYALMGAVGGTVAIATLTVISIDRYNVVVYPLNPNRSTTKLKCYFLIAFTWAYGLLFASFPALEIGLS
UV7_droMel DLSYIAKVNPFWLQFEPPKSSTFLIMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLI-KCPIAIYNNI--KEGPALGDIACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIILLIWCYSFLFAVMPALDIGLS
UV7_droYak DLSYIAKVNPFWLQFEPPKSSTFLVMAALYFLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLI-KCPIAIYNNI--KEGPALGDIACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIILLIWCYSFLFAVMPALDIGLS
UV7_droAna DLSYIAKVNPFWLQFEPPHSSTFLAMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLV-KCPIAIYNNI--KEGPALGDVACRIYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIILLIWCYSFLFAVMPALDIGLS
UV7_droPse DLSYIARVNPFWLQFEPPKSSTFYLMAALYCLISVVGCVGNAFVIFMFANRKSLRTPANILVMNLAICDFLMLV-KCPIAIYNNI--KEGPALGDAACRIYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLIIFLIWSYSFLFAVMPALDIGLS
UV7_droWil DLSYIAKVNPFWLQFEPPRSSTFYIMAALYCLISVVGCIGNAFVIFMFSNRKSLRTPANILVMNLAICDFLMLV-KCPIAIYNNI--KEGPALGDIACRIYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRCSRLRSYLVIVMIWCYSFLFAIMPALDVGLS
UV7_droMoj DLTYIAKVNPFWLQFEPPDTSTFYIMAALYCLISVVGCVGNAFVIFMFGSRKSLRTPANILVMNLAICDFLMLV-KCPIAIYNNI--QEGPALGDAACRLYGFVGGLSGTCAIGTLTAIALDRYNVVVHPLQPLRRYSRLRSYLIIFAIWCYSFLFAVMPALDVGLS
UV7a_acyPi TDDYIKLINSHWLKFMPPNPTSHYVLGLLYTVIMVFGCTGNSLVIFMYFKCRSLQTPANMLIINLAVSDFIMLA-KASVFIYNSY--YLGPALGKLGCQVCGFLGGLTGTVSIMTLAAISLDRYYVIVCPLKAAVKTTKQRARIWIGLIWIYGFSFSIVPVLDLGYS
UV7b_acyPi TDDYMKLINSHWFKFMPPNATSHYILGFLYSVIMVLGCFGNSLVIFMYIKCKSLQTPANVLIMNLAVSDFIMLA-KTPVFIYNSF--YQGPTLGKLGCQIYGFFGGLTGTVSIMTLAAISLDRYYVIVHPLNAAVKTTKQRARVWIGLIWIYGFLFSIIPVMDLGYN
UV7_rhoPro TEEYLKLVNTHWFEYPPPNKQIHYIFAAVYFLVMLVGVSGNLLVIFMILRFRTLRTSSNILILNLAVSDFLMVA-KMPVFIYNSF--YFGPVLGEMGCHFYGFIGGLSGTASILTLAAIAMDRYLGIAHPLNFNQGRAKKRTIVWITFIWVYSITFASIPLSHIGVK
UV7_pedHum DDEYLYKINKYWMKFPPPSPMSHYFMGIIYSVIMVVGVFGNFLIIYLFLRKRSLRTPSNVFIFNLAVSDSLLLL-KMPVFIINSF--YLGPALGNLGCSAYGFVGGLTGTVSIMTLAAIAFDRYQVIVHPLE---RKTKAAVYFQILLIWIYAIFFSIIPLLDVGLN
UV7_ixoSca TEEYLKLVNTHWFEYPPPNKQIHYIFAAVYFLVMLVGVSGNLLVIFMILRRRRIRSQANLLVFNLALSDLLMVL-EIPLLVYNSL--KLRPALGVWGCQLYGLMGGLSGTSAIFSIAALSLERYLALGRPRDPFARLTRSRAFALSLSSWIYALCFSAWPLLGV-TS
UV5_anoGam PPEELVHIPEHWLQFPEPEASLHYLLGLLYIAFTIFSLVGNGLVIWIFIAAKSLRTPSNVFVINLAICDFFMMA-KTPIFIYNSF--TKGFTLGNLGCQIFGFVGSLTGIGAGATNALIAYDRYNTITRPFE--GRLTQTKAIIFICLIWAYTIPWGVLPLLEI-WG
UV5_nasVit PPEELVHIPEHWLVYPEPNPALHYLLALLYILFTFVALLGNGLVIWIFCAAKSLRTPSNMFVVNLAICDFMMML-KTPIFIYNSF--HTGFALGNLGCQIFSFIGSLSGIGASITNAAIAYDRYSTIARPLD--GKLSRGQVMMLIVLIWMYTIPWALMPSMGV-WG
UV5_apiMel PAEELIHIPEHWLVYPEPNPSLHYLLALLYILFTFLALLGNGLVIWIFCAAKSLRTPSNMFVVNLAICDFFMMI-KTPIFIYNSF--NTGFALGNLGCQIFAVIGSLTGIGAAITNAAIAYDRYSTIARPLD--GKLSRGQVILFIVLIWTYTIPWALMPVMGV-WG
UV5_diaNig PAEELIHIPEHWLTYPAPDAFSYYILGMLYVAFCFIALIGNGLVIWVFSSAKTLRTPSNIFVINLALYDFIMML-KTPIFIYNSF--NLGFGLGQLGCQIFAFMGSVSGIGAAATNACIAYDRYRVIARPFD--SKMSIKGATLLVLLVWMWALPWAILPLLEI-WG
UV5_lucCru PKSELHHIPEHWLVYPEPEASIHYLLGIVYIFICFMGIVGNGLVLWIFSTSKSLKTASNMFVVNLAFCDFIMMM-KMPIFVYNSF--NRGYALGHIGCQIFGFVGSLSGIGAGMTNAFIAYDRYATISNPLE--GKLTRTKALIMIFIIWGYTFPWAVLPMFEV-WC
UV5_triCas PKDELIHIPQHWLVYPEPEASMHFLLALIYIGFFIMATIGNGLVIWIFSTSKSLRTASNMFVVNLAICDFAMMI-KTPIFIYNSF--YRGFALGHLGCQIFAFIGSLSGIGAGMTNACIAYDRYTTITRPFD--GKITRTKALVMIIFVWGYTIPWAVMPLLEI-WG
UV5_rhoPro SPEDLKHIPEHWLSYPEPEPILNYALGVLYIFFMLIALIGNGLVIWIFSTAKTLRTPSNIFVVNLAICDFLMMS-KTPIFIYNSF--KLGYALGHRACQIFALLGSFSGIGASATNAVIAYDRYRVIATPFA--PKLSRTKAVLYLALVWAYVTPWALLPLFEQ-WS
UV4_droMel PPDQIQYIPEHWLTQLEPPASMHYMLGVFYIFLFCASTVGNGMVIWIFSTSKSLRTPSNMFVLNLAVFDLIMCL-KAPIFIYNSF--HRGFALGNTWCQIFASIGSYSGIGAGMTNAAIGYDRYNVITKPMN--RNMTFTKAVIMNIIIWLYCTPWVVLPLTQF-WD
UV3_droMel PPEELRHIPEHWLTYPEPPESMNYLLGTLYIFFTLMSMLGNGLVIWVFSAAKSLRTPSNILVINLAFCDFMMMV-KTPIFIYNSF--HQGYALGHLGCQIFGIIGSYTGIAAGATNAFIAYDRFNVITRPME--GKMTHGKAIAMIIFIYMYATPWVVACYTET-WG
UV5_manSex TGDDLAAIPEHWLSYPAPPASAHTALALLYIFFTFAALVGNGMVIFIFSTTKSLRTSSNFLVLNLAILDFIMMA-KAPIFIYNSA--MRGFAVGTVGCQIFALMGAYSGIGAGMTNACIAYDRHSTITRPLD--GRLSEGKVLLMVAFVWIYSTPWALLPLLKI-WG
UV5_papXut TGEDLAAIPEHWLSYPAPPASAHTMLALVYVFFTAAALIGNGLVIFIFSASKSLRTPSNLLVVQLAVLDFLMML-KAPIFIYNSI--KRGFASGVIGCQIFAFMGSVSGTAAGLTNACIAYDRHSTITRPLD--GRLSRGKVLLMMVCVWLYTAPWAILPQLQI-WG
UV5_acyPis QAEDLIHIPEHWLKYQEPSSLQHYYLAFMYTIFMFVALFGNGLVIWVFCVAKPLRTPSNIFVINLALCDFVMMA-KAPIFILGSI--NRGYQ-GHFLCQLFGTAGAFSGIGASATNAAIAYDRFSTIAKPFD--GRMTYGRAFFLIICIWTYTLPWGLLPLTEK-WN
UVB_acyPis TPEDLTHIPEHWLSYPEVRSLYHYILAFSYTILFCLGVIGNGLVLWIFCVSKPLRTPSNLFVLNLALCDFSMVL-VLPILIYDSI--DHKYP-GHLQCQIFALCGSISGIGAGATNAAIAYDRYSTIAKPFE--GRMTYGKALILIICIWIYVLPWCLLPLTEK-WN
UVB_megVic TPEDLTHIPEHWLSYPEVRSLYHYILAFSYTILFCIGVIGNGLVLWIFCVSKPLRTPSNLFVLNLALCDFSMVL-VLPILIYDSI--DHKYP-GHLQCQIFALCGSISGIGAGATNAAIAYDRYSTIAKPFE--GRMTYGKALILIICIWIYVLPWCLLPLTEK-WN
UV5_dapPul PEDYMSYVHPYWKTFEAPNPFLLYMIGFLYTIFMFCCVAGNGVVIWIFTNCKSLRTPSNMLVVNLAILDMLMML-KSPVMIINSY--NEGPIWGKLGCDVFGLMGSYNGIGSAVNNAAIAYDRHRTISRPLD--GKLSRKQVTLMIVAIWAWATPFSVMPFLGI-WG
UV5_braKug PAEYMEFVHPHWKQFEAPNPFLHYMLGVFYIIFMFCSLIGNGVVIWVFASAKSLRTPSNLFVINLAVLDFLMML-KTPVFIVNSF--NEGPIWGKTGCDFFALLGSYAGIGGATTNAAIAFDRYRTIAHPFD--GKLSRGQAITLCMLCWLYATPFSLMPFFGI-WG
UV5_triLon PKDYMEYVHPHWQTFEAPNPFLHYLLGVLYIGFMFCALVGNGVVIWIFSSAKSLRTPSNMFVINLAVLDFIMMM-KTPVFIVNSF--NEGPIWGKFGCDLFALMGSYSGIGGAMTNAAIAFDRYRTIARPFD--GKLSRGKVLTICAGIWLWATPFSLMPLFGI-WG
UV5_triGra PKDYMDYVHPHWQTFEAPNPFLHYLLGVLYIGFMFCALVGNGVVIWIFSSAKSLRTPSNMFVINLAVLDFIMMM-KTPVFIVNSF--NEGPIWGKFGCDMFALMGSYSGIGGAMTNAAIAFDRYRTIARPFD--GKLSRGKVLTICAGIWLWATPFSLMPLFGI-WG
UV5_pedHum DPSELVHIPDHWFNFSAPHPLSNYLLGFLYFIFFVISCTGNGIVIWIFTTSKNLRTASNVFVVNLAIFDFIMMA-KTPIMIYNSM--NLGFECGFVWCQIFASAGALSGIGASITNTCIAYDRCETITNPLQ---KSGKKKAFLLAAFTWIYALPWAVLPFLEI-WG
UVB_anoGam PPEEQYLVHDHWKGFPSPPYYMHLMLAMIYFVLMNTSLIGNGIVLWIFGTSKSLRNGSNMFIINLAIFDLLMMC-EMPMFLVNSF--SERLVGYGVGCSVYAALGSMSGIGGAISNAVIAFDRYRTISNPLD--GRLSRVQAGLLICLTWLWTMPFTLLPLFEI-WG
UVB_manSex PEEHQDLVHDHWRNFPAVSKYWHYVLALIYTMLMVTSLTGNGIVIWIFSTSKSLRSASNMFVINLAVFDLMMML-EMPLLIMNSF--YQRLVGYQLGCDVYAVLGSLSGIGGAITNAVIAFDRYKTISSPLD--GRINTVQAGLLIAFTWFWALPFTILPAFRI-WG
UVB_nasVit PPEHIDIVHPHWRGFLAPGKYWHIGLALIYFMLLVLSFVGNGCVVWIFSTSKVLRTPSNLFIINLALFDLVMAL-EIPMLIINSF--IERMIGWGLGCDIYAALGSVSGIGSAITNAAIAYDRYRTISCPID--GRLNGKQAAVMVAFTWFWTMPFTILPFAKI-WG
UVB_apiMel PPEYSDLVHPHWRAFPAPGKHFHIGLAIIYSMLLIMSLVGNCCVIWIFSTSKSLRTPSNMFIVSLAIFDIIMAF-EMPMLVISSF--MERMIGWEIGCDVYSVFGSISGMGQAMTNAAIAFDRYRTISCPID--GRLNSKQAAVIIAFTWFWVTPFTVLPLLKV-WG
UVB_diaNig PAEHIELVHSHWRGYEAPSKYWHYWFAFMYFCIMIMSCLGNGIVLWIFATTKSLRTPSNMFVVNQALLDLLMMI-EMPMFVLNSL-FYQRPIGWEMGCDIYALLGAVSGIGSAINNAAIAYDRYRTISFPLD--GRLQFGHALAFIVGVWSWAMPFSLLPLLKV-WG
UV5B_droMe PAEYQHMVHAHWRGFREAPIYYHAGFYIAFIVLMLSSIFGNGLVIWIFSTSKSLRTPSNLLILNLAIFDLFMCT-NMPHYLINAT--VGYIVGGDLGCDIYALNGGISGMGASITNAFIAFDRYKTISNPID--GRLSYGQIVLLILFTWLWATPFSVLPLFQI-WG
UV5_plePay NAAPDIYVPDYWKQFRAPAPYLHYMLGFFYICLMSIAVVGNAIVMYIFFSAKTLRTPTNMFVIGLAMADLLMMS-KTPVFIYNCF--HLGPVFGQIGCDIYGIVGTYSGIGSAFCNAIIAYDRYRVIVHPFSK-SGMSITKAIAFLVIIYLYITPFAILPALKI-WS
UV5_hasAda NAAPDILVPDYWKQFRAPAPYLHYILGCLYICLMSVALIGNAIVIYIFSVSKSLRTPTNMFVIGLAMADLLMMS-KTPVFIYNCF--HLGPVFGQLGCDIYAIVGTYSGIGSAFCNAVIAYDRYRVIVHPFSK-SGMTMTKAIAILVIVYLYITPFAILPALKI-WS
LWS_anoGam PPEIMHLVDPHWSQFPPMNPLWHSIIGFVIFVLGVVSIIGNGMVIYIFSTAKSLRTPSNLFIVNLALSDFLMMGTNAFTMVYNCW--FETWSLGLLMCDLYAFFGSLFGCCSIWTMTMIALDRHNVIVHGLSG-KPLTNTGAILRILLCWLIGVVWGILPMLG--WN
LWS_rhoPro PPEMLSMVDAHWYQFPPLNPLWHGILGFVIGVLGIISIVGNGMVIFIFSSTKTLRTPSNLLVVNLAFSDFLMMFTMSPPMVINCY--NETWVLGPLMCELYGMLGSLFGCASIWTMTMIALDRYNVIVKGISA-KPMTNKTAMLRILLVWAFSIMWTVFPFFG--WN
LWS_schGre PPEMLYLVDPHWYQFPPMNPLWHGLLGFVIGVLGVISVIGNGMVIYIFSTTKSLRTPSNLLVVNLAFSDFLMMFTMSAPMGINCY--YETWVLGPFMCELYALFGSLFGCGSIWTMTMIALDRYNVIVKGLSA-KPMTNKTAMLRILFIWAFSVAWTIMPLFG--WN
LWS_lucCru PPDMLHLIDAHWYQYPPLNPLWHAILGFMIGVLGCISVTGNGMVIYIFSTTKSLRSPSNLLVVNLAFSDFLMMFTMAPPMVINCY--NETWVWGPLFCQIYGMLGSLFGCTSIWTMTMIALDRYNVIVKGLSA-KPLTKQGALIRIFLVWVFSIGWTIAPVFG--WN
LWS_triCas LPDMLHLVDAHWYQFPPMNPLWHGILGFVIGVLGFVSIVGNGMVIYIFSSTKALRTPSNLLVVNLAFSDFLMMLCMSPAMVINCY--NETWVLGPLVCELYGMSGSLFGCASIWTMTFIALDRYNVIVKGLSA-QPLTKKGAMLRILIIWVFSTLWTIAPFFG--WN
LWS_manSex PPDMMHMIDPHWYQFPPMNPLWHALLGFTIGVLGFVSISGNGMVIYIFMSTKSLKTPSNLLVVNLAFSDFLMMCAMSPAMVVNCY--YETWVWGPFACELYACAGSLFGCASIWTMTMIAFDRYNVIVKGIAA-KPMTSNGALLRILGIWVFSLAWTLLPFFG--WN
LWS_papXut TPDMMHLIDPHWYQFPPMNPMWHGLLGFTIGVLGFISITGNGMVVYIFTSTKSLKTPSNLLVVNLAFSDFLMMLCMAPPMLINCY--YETWVFGPLACELYACAGSLFGSISIWTMTMIAFDRYNVIVKGIAA-KPMTINGALLRILGIWLFSLAWTIAPMLG--WN
LWSb_apiMe PPDMLHLIDANWYQYPPLNPMWHGILGFVIGMLGFVSVMGNGMVVYIFLSTKSLRTPSNLFVINLAISDFLMMFCMSPPMVINCY--YETWVLGPLFCQIYAMLGSLFGCGSIWTMTMIAFDRYNVIVKGLSG-KPLSINGALIRIIAIWLFSLGWTIAPMFG--WN
LWS_homCoa PPEMLYLVDAHWYQFPPMNPLWHSLLGFAMVVLGFIAVTGNGMVVYIFSCTKALRTPSNLLVVNLAFSDFLMMFTMAPPMVLNCY--YETWVLGPFMCELYAMFGSILGCTSIWTMVMIANDRYNVIVKGLSA-KPMTIKSALARILFCWAHSLIWCLAPFLG--WG
LWSa_nasVi PPEIHHMIDPYWYQFPPMNPLWYGILGFVIGCLGCISVAGNGMVVYIFASTKSLRTPSNLLVINLAFSDFCMMFTMSPPMVINCY--YETWVFGPLMCEIYALCGSIFGCGSIWTMCMIAFDRYNVIVKGLSA-KPMTINGSLLRILGIWLMASIWTIAPMFG--WN
LWS_acyPis PADMMHLIDPSWYQFPPMESMWYKWLGVTIFFLGILSVVGNGMVIYIFTCTKNLRTPSNLLIVNLAFSDFCLMFTMCPAMVWNCF--YETWMFGPFACELYAMFGSLFGVTSIWTMVFIALDRYNVIVKGLSA-KPMTTKLALLQIFCIYLHGLFWTLTPFFG--WS
LWSb_nasVi PPEMLHLVGPHWYQFPPLWPIWHKLLGVVMIFIGVLGWCGNGMVVYIFLVTPSLRTPSNLLVINLAFSDFVMMIIMSPPMVVNCW--YETWILGPLMCDIYALIGSLCGGASIWTMTAIAYDRYNVIVKGMSG-TPLTIPRALVQIVLIWTHGLIWAMLPLFG--WN
LWSa_apiMe PEEMLHLIDLYWYQFPPLDPLWHKILGLVMIILGIMGWCGNGVVVYVFIMTPSLRTPSNLLVVNLAFSDFIMMGFMCPPMVICCF--YETWVLGSLMCDIYAMVGSLCGCASIWTMTAIALDRYNVIVKGMSG-TPLTIKRAMLQILGIWLFGLIWTILPLVG--WN
LWS6_droMe PAEIMHMVDPYWYQWPPLEPMWFGIIGFVIAILGTMSLAGNFIVMYIFTSSKGLRTPSNMFVVNLAFSDFMMMFTMFPPVVLNGF--YGTWIMGPFLCELYGMFGSLFGCVSIWSMTLIAYDRYCVIVKGMAR-KPLTATAAVLRLMVVWTICGAWALMPLFG--WN
LWS_meoOer PENMLHMIHSHWYQFPPLNPMWYGILAFVVTVVGLCSICGNFVVIWVFMNTKALRSPANTLVVSLAVSDFIMMACMFPPLVLNCY--WGTWIFGPLFCEVYAFIGNTVGCASIGNMIFITFDRYNVIVKGISG-TPLSQKNTTLQVLFVWICSIMWCVFPFFG--WN
LWS1_droMe TPDMAHLISPYWNQFPAMDPIWAKILTAYMIMIGMISWCGNGVVIYIFATTKSLRTPANLLVINLAISDFGIMITNTPMMGINLY--FETWVLGPMMCDIYAGLGSAFGCSSIWSMCMISLDRYQVIVKGMAG-RPMTIPLALGKIAYIWFMSSIWCLAPAFG--WS
LWS2_droMe LPDMAHLVNPYWSRFAPMDPMMSKILGLFTLAIMIISCCGNGVVVYIFGGTKSLRTPANLLVLNLAFSDFCMMASQSPVMIINFY--YETWVLGPLWCDIYAGCGSLFGCVSIWSMCMIAFDRYNVIVKGING-TPMTIKTSIMKILFIWMMAVFWTVMPLIG--WS
LWS_limPol PKEMLYMIHEHWYAFPPMNPLWYSILGVAMIILGIICVLGNGMVIYLMMTTKSLRTPTNLLVVNLAFSDFCMMAFMMPTMTSNCF--AETWILGPFMCEVYGMAGSLFGCASIWSMVMITLDRYNVIVRGMAA-APLTHKKATLLLLFVWIWSGGWTILPFFG--WS
LWS_ixoSca PDEMLYMVHPHWYNFKPMNPLWHSLLGFAMVILGVISVVGNSMVIYIMTTSKSLRSPTNMLVVNLAFSDWCMMAFMMPTMAANCF--AETWILGPFMCEVYGMVGSLFGCGSIWSMVMITLDRYNVIVRGVAA-APLTHKRAALMIFFVWFWALTWTLLPFFG--WS
LWS2_plePa PKEILHMIHDHWYQFPPLNPLWHSLLGIAMILLGIVSVIGNGMVMYLMNTTKSLKTPTNMLIVNLAFSDFCMMAFMMPTMAANCF--AETWILGPFMCEIYGMAGSLFGCVSIWSMVMIAFDRYNVIVRGMNA-EPLTTKKAAAQIFLIWAWAIMWTVLPFFG--WS
LWS2_hasAd PKEILHMIHDHWYQFAPLNPLWHSLLGIAMIILGIVSVIGNGMVIYLMSTTKSLKTPTNMLIVNLAFSDFCMMAFMMPTMAANCF--AETWILGPLMCEIYGMAGSLFGCVSIWSMVMIAFDRYNVIVRGMSA-EPLTTKKAAAQIFFIWTWATTWTLFPFFG--WS
LWS1_plePa PEDMLYMIHEHWYKYPPMESTMHYLLGITIILIGIISVSGNSIVIYLMLSVKSLRTPANFLVTSLAVSDGGMLAFMAPTMPINCF--AQTWVLGPFMCELYGMVGSLFGSASIWNMVMITLDRYNVIVRGMSG-KPLTKVGALLRIIFVWVWSLGWTIAPMYG--WS
LWS1_hasAd PEDMLPMIHEHWYKFPPMETSMHYILGMLIIVIGIISVSGNGVVMYLMMTVKNLRTPGNFLVLNLALSDFGMLFFMMPTMSINCF--AETWVIGPFMCELYGMIGSLFGSASIWSLVMITLDRYNVIVKGMAG-KPLTKVGALLRMLFVWIWSLGWTIAPMYG--WS
BCRa_hemSa PDRVKHMVLDHWYNYPPVNPMWHYLLGVVYLFLGVISIAGNGLVIYLYMKSQALKTPANMLIVNLALSDLIMLTTNFPPFCYNCF-SGGRWMFSGTYCEIYAALGAITGVCSIWTLCMISFDRYNIICNGFNG-PKLTQGKATFMCGLAWVISVGWSLPPFFG--WG
BCRb_hemSa RPEIKPYVHQHWYNYPPVNPMWHYLLGVIYLFLGTVSIFGNGLVIYLFNKSAALRTPANILVVNLALSDLIMLTTNVPFFTYNCF-SGGVWMFSPQYCEIYACLGAITGVCSIWLLCMISFDRYNIICNGFNG-PKLTTGKAVVFALISWVIAIGCALPPFFG--WG
BCR_porPel RPEIKPYVHQHWYNYPPVNPMWHYLLGVIYLCLGFISIIGNGMVIYLFAKCQALRTPANILVVNLALSDLIMLTTNVPFFTYNCF-NGGVWMFSATYCEIYGCLGAITGVTSTWLLCMISFDRYNIICNGFNG-PKLTNGKAIILAFISWAISVGFGIAPLFG--WG
BCR_triGra PSDMKTMVHSHWNKFPPVNPMWHYLLGMVYIILGTVSIAGNSLVISLFTKTKELRTPANMFVVNLAFSDLCMMITQFPMFVYNCF-NGGMWLFGPFLCELYAATGAVFGLCSICTLACIAFDRYNLIVKGMSG-PKMTSKRATILIAFCWAYAIGWSLPPFFG--WG
BCR2_triLo PSDMKTMVHSHWSKFPPVNPMWHYLLGLVYIVLGTVSIAGNSLVISLFTKTKELRTPANMFVVNLAFSDLCMMITQFPMFVYNCF-NGGMWLFGPFLCELYAATGAVFGLCSICTLACIAYDRYNLIVKGMSG-PKMTSKRATILIAFCWSYAIGWSLPPFFG--WG
BCRa_dapPu PDDMKEFIHPHWNKFPPVNPMWHYLLGVIYVILGITSVTGNSLVVHLFAKTRDLRTPANMFVINLAFSDLCMMITQFPMFVFNCF-NGGVWLFGPLFCELYACTGSIFGLCSICTMAAISYDRYNVIVNGMNR-RRMTYGRAGGLILFCWIYAIGWSIPPFVG--WG
BCR_limPol PENIKHLISDHWSKFPAVNPMWHYLLGLIYIVLGIASLTGQSVVLYLFAKTKPLRTPANMLIVNLAFSDFMMMITQFPVFIINCL-GGGAWQLGPLLCEITGFAGGLFGYGSIVTLAVISIDRYNVIVRGFSA-SPLTHARSAVFILVIWAWTLGWALPPFFG--WG
BCR2_braKu PADVIAMTHAHWKQFPPSNPAWNYLFGVIYFFLWIVNHIGNGLVIWIFLKTKSLRTPSNMLIVNLAIADFFMMLTQSPLYIISAF-TSRWWIWGHFWCRFYGYTGGITGIAAIFTMVFIGYDRYNVIVKGMNG-TKITKGMAFIMILWTWIYANAFCLPAMLEV-WG
BCR3_braKu PADIVALTHAHWKKFPPSNPAWNYLFACLYFFLWVINHIGNGLVIKIFLKTKSLRTPSNMLIVNLAIADFFMMLTQSPLFIISAF-SSRWWIWGHFWCRFYGYTGGITGIAAIFTLVFIGYDRYNVIVKGMSG-KRISKGMAFGMIVWTWVYANVFCLPPMLQV-WG
BCR1_triGr PEDVRAFLHPHWHNFPATHPAIYYLFGLVYLVLGVTSVGGNYLVLRIFTKFQELRRPSNVLVINLALSDMLLMLTLFPECVYN-FLSGGPWRFGDLGCQIHAFCGALFGYNQITTLVFISYDRFNVIVRGMGG-TPLTYARVSAMVAFSWLWATGWSVAPLVG--WG
BCR2_triGr PLDMHHLLHSHWDAYPPADPRIHYLLGMLYFFLGIAACMGNVLVLHIFGKHKNLRSPTNTLLMNLAFCDLMIFIGLYPEMLGNIFMNDGTWMWGDVACRIHAWFGLVFGFGQMQTLMYMSIDRYNVIVKGLSA-QPLTYKKVTQWLAQVWIVSLFWGTAPFFG--FG
BCR1_triLo PLDMHHLLHSHWDSYPPADPRIHYLLGMLYFFLGIAACVGNVLVLHIFGKHKNLRSPTNTLLMNLAFCDLMIFIGLYPEMLGNIFMNDGTWMWGDIACRLHAWFGLVFGFGQMQTLMYMSIDRYNVIVKGLSA-QPLTYKKVTQWLAQVWIVSLFWGTAPFFG--FG
BCR3_triGr PENVRYMVHLHWEKFPPPDPRVHTALGALYLIMGVMSAVGNVLVLYIFGKYKSLRSPTNVLVMNLAFCDLGLFVGLYPELLGNIFINNGPWMWGDVACKIHAWCGLAFGFGQMQTLMFVSMDRYYVIVKGLKA-PPLTYWKVSVWLAMVWIVSIFWATSPFFG--FG
Consensus p......!..hW..%ppp.p..hy.lg..y......s..GNg.Vi.if...ksLRtpsN.l!.NLA..Df.$m....P....N.. .......g...C.i%a..G.l.G..si.t...Ia.DR%nv!v.p......lt...a...i...W.....w...P.....w.
Special res .......................................................<---------HEK region-------->...................................................K............N.PRYR..........
UV7 diagnos .......................................................<----16aa HEK region del---->.............................................................................p.l
UV diagnos .............................................................................................................................................D.........RF...........
Location E2E2E2E2E2E2E2E2E2E2E2E2EM5M5M5M5M5M5M5M5M5M5M5M5M5M5M5C3C3C3C3C3C3C3C3C3C3C3C3C3C3C3C3C3CM6M6M6M6M6M6M6M6M6M6M6E3E3E3E3E3E3EM7M7M7M7M7M7M7M7M7M7M7CTCTCTCTCTCTCTCTC
UV7_aedAeg RYTPEGFLTACSFDYLERT-RDARLFMFLYFIFAWVVPIIAITFCYIQILRVVIGAN---------SIQSSKNKSKT-------EVKLAGVVIGIIGLWFIAWTPYAIVAMMGVFGYESL--LSPLGSMVPAILAKTAACIDPYFYAMNHPRYRQELRKMFGLN
UV7_culQui RYTPEGFLTACSFDYLDRG-WDARVFMFMYFVFAWVIPFLTISYCYVAILRVVVGAG---------SIQSSKNKNKQ-------EVKLAGVVIGIIGLWFIAWTPYAVVAMLGVFGYEHL--LTPLGSMIPAILAKTASCIDPYFYAMNHPRFRQELRKMFGKE
UV7_anoGam RYTAEGYLTACSFDYLDRT-YKARVFMFVYFVFAWLIPFAIISYCYARILIAVINAN---------AIQSSKSKNKT-------EVKLAGVVVGIIGLWFAAWTPYAVVAMMGVFGYEQY--LTPLNSMIPAVFAKIAASIDPYFYAMNHPRYRQMLERMFCNR
UV7_droMel VYVPEGFLTTCSFDYLNKE-MPARIFMALFFVAAYCIPLTSIVYSYFYILKVVFTAS---------RIQSNKDKAKT-------EQKLAFIVAAIIGLWFLAWSPYAIVAMMGVFGLERH--ITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFYGR
UV7_droYak VYVPEGFLTTCSFDYLNKE-TPARIFMALFFVAAYCIPLTSIVYSYFYILKVVFTAS---------RIQSNKDKAKT-------EQKLAFIVAAIIGLWFLAWSPYAIVAMMGVFGLERH--ITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFYGR
UV7_droAna VYVPEGFLTTCSFDYLNKE-TPARIFMALFFVAAYCFPLTAIVYSYFYILKVVFSAG---------RIQSNKDKAKT-------EQKLAFIVAAIIGLWFLAWSPYAVVAMMGVFGLEKH--ITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFYGR
UV7_droPse VYVPEGFLTTCSFDYLNKE-TPARIFMALFFVAAYCVPLTTIVYSYFYILKVVFTAS---------RIQSNKDKAKT-------EQKLAFIVAAIIGLWFLAWSPYAIVAMMGVFGQERH--ITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLFFGR
UV7_droWil VYVPEGYLTTCSFDYLNKE-TPARIFMALFFVAAYCVPLTCIMFSYFYILKVVFTAN---------RIQSNKDKAKT-------EQKLTFIVAAIIGLWFLAWSPYAVVAMMGVFGLEQH--ITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLIYGR
UV7_droMoj VYVPEGFLTTCSFDYLNKE-TPARIFMALFFVAAYCIPLASIVYSYFYILKVVFTAN---------RIQSSKDKAKT-------EQKLTFIVAAIIGLWFIAWSPYAIVAMMGVFGQEQH--ITPLGSMIPALFCKTAACVDPYLYAATHPRFRVEVRMLMYGR
UV7a_acyPi RYVSEGYLTSCSFDYLSDN-DQDKRFILVFFTAAWCIPFTIILYCYVNILMAVWMTT----EIVTSRVGQQEEKRKT-------DIRLGYMVIGALALWFVSWTPYAVVALLGVFDLKEY--ISPLSSMIPALFCKAASCTDPWFYAITHPRFKKELMKLLTKS
UV7b_acyPi RYVPEGYLTSCSFDYLSDD-NQEKGFILVFFTAAWCIPFTTISYCYIKILRAVWMTS----EMAASRFGQEEEKRKT-------EIRLGYVVVGVIMLWFVSWTPYAMVALLGVFDRKDY--ITPLSSMIPAVLCKAASCMDPWIYAITHPRFKNELTKLMSRK
UV7_rhoPro TYVPEGFLTSCSFDYLSTD-IQNRCFIFIYFVAAWCLPLLVIITSYVGICREVLRVS----LI---RKGQEREQRKR-------EAKLSAILALATFLWFLSWTPYAAVALLGIFGYKNH--ITQLASMIPALFCKTAACVNPFIYGLNHPRLRQQLLKLCCKK
UV7_pedHum KYVPEGYLTSCSFDYLTQD-TASRLTIFVFFVAAWIVPLSIILGSYMALYKVVLKARGTHFNTVMTRHCKDIEIQRP-------ELKAAVTVICIVCLWTLSWTPYAVVALLGITGNEKY--ISPMSSMIPALFCKTASCIDPFVYAATNRRFRNELKRKYRKR
UV7_ixoSca PYVPEGFLTSCSFHFLSDA-TSDRCFVWIFFVAAWCVPLVFVTTCYSGILVTVIRS----------RKALAQESRRS-------ELRVAKVSLALVLLWTVAWTPYAIVALLGITGRRNL--LTPWGSMAPAMFCKSAAVLDPFVYGLSHPSFRRELAIMLPCL
UV5_anoGam RYVPEGFLTSCTFDYLSGT-FDTRLFVASIFTFSYVLPMSLIIYYYSQIVSHVVNHEKSLREQAKKMNVESLRSNQNQK-DASVEIRIAKAAITVCFLFVASWTPYAVLALIGAFGDKSL--LTPGVTMFPACACKFVACLDPYVYAISHPRYRIELQKRLPWL
UV5_nasVit RFVPEGFLTSCTFDYITDS-DEIRYFVGTIFTFSYAIPMTLIIYFYSQIVGHVVNHEKALREQAKKMNVESLRSGQNKD-QASAEVRIAKVALTICFLFVAAWTPYGVMSLIGAFGNKSL--LTPGVTMIPACCCKAVACLDPYVYAISHPRYRLELQKRMPWL
UV5_apiMel RFVPEGFLTSCSFDYLTDT-NEIRIFVATIFTFSYCIPMILIIYYYSQIVSHVVNHEKALREQAKKMNVDSLRSNANTS-SQSAEIRIAKAAITICFLYVLSWTPYGVMSMIGAFGNKAL--LTPGVTMIPACTCKAVACLDPYVYAISHPKYRLELQKRLPWL
UV5_diaNig RYAPEGYLTSCSFDYLTDT-PENHMFVLCIFICSYVIPMSLIIYFYSQIVSHVVNHEKALKEQAKKMNVDSLRSNQQQN-QTSAEIRIAKVAIGICFLFVASWTPYAVLALIGAFGNKAL--LTPGVTMIPACTCKAVACLDPYVYAISHPRYRAELQKRLPWL
UV5_lucCru RFVPEGFLTSCTFDYLTDT-FDNDMFVAVIFICSYVIPMSMIIYFYSQIVKHVMHHEKALRDQAKKMNVESLRSNQSLQ-SQSIEIKIAKVAIMVCFLFVASWTPYAVLALIGGFGDQSL--LTPGVTMVPALACKFVACLDPYVYALSHPRYRMELQKRLPWL
UV5_triCas RFAPEGFLTACSFDYLTDT-FDNHMFVTSIFICSYVIPMSMIIYFYSQIVSKVFSHEKALREQAKKMNVESLRSNQSQQASQSAELRIAKAAIAICSLFVASWTPYAVLALIGAFGDQSL--LTPGVTMVPACACKFVACLDPYVYAISHPKYRLELQKRLPWL
UV5_rhoPro RFVPEGFLTSCTFDYLTPT-SEIRNFVTVMFFICYVFPMSLIIYFYSQIVSHVIIHEHNLREQAKKMNVESLRSNANMH-TQSAEIRIAKAAITICFLFVASWTPYAVLALIGAYGNQDL--LTPAVTMIPACACKAVACVDPYVYAISHPRYRQELSKKFPWL
UV4_droMel RFVPEGYLTSCSFDYLSDN-FDTRLFVGTIFFFSFVCPTLMILYYYSQIVGHVFSHEKALREQAKKMNVESLRSNVDKS-KETAEIRIAKAAITICFLFFVSWTPYGVMSLIGAFGDKSL--LTPGATMIPACTCKLVACIDPFVYAISHPRYRLELQKRCPWL
UV3_droMel RFVPEGYLTSCTFDYLTDN-FDTRLFVACIFFFSFVCPTTMITYYYSQIVGHVFSHEKALRDQAKKMNVESLRSNVDKN-KETAEIRIAKAAITICFLFFCSWTPYGVMSLIGAFGDKTL--LTPGATMIPACACKMVACIDPFVYAISHPRYRMELQKRCPWL
UV5_manSex RYVPEGYLTSCSFDYLTNT-FDTKLFVACIFTCSYVFPMSLIIYFYSGIVKQVFAHEAALREQAKKMNVESLRANQGGS-SESAEIRIAKAALTVCFLFVASWTPYGVMALIGAFGNQQL--LTPGVTMIPAVACKAVACISPWVYAIRHPMYRQELQRRMPWL
UV5_papXut RYVPEGFLTSCTFDYLTTT-FDNKLFVASMFVCVYIFPMIAILYFYSGIVKQVFAHEAALREQAKKMNVDSLRSNQNAA-AESAEIRIAKAALTVCFLYVASWTPYGVMSLIGAFGDQNL--LTPGVTMIPALACKGVACIDPWVYAISHPKYRQELQKRMPWL
UV5_acyPis RYVPEGYLTSCTFDYLSPT-DETRAFVGIMFVICYVIPVSLVIFFYSQIVSHVFNHEKALREQAKKMNVESLRSNQDAN-AQSAEVRIAKAAITICCLFIASWTPYAVVAMIGAFGDRSL--LTPGITMIPAIFCKTVACFDPYVYAISHPRYRLELSKRVPCL
UVB_acyPis RFVPEGFLTSCSFDYLTPT-EETKAFVGTMFVICYVIPMSFIIYFYSQIVCHVFNHEKALREQAKKMNVESLRSNQDAN-AQSAEVRIAKAAITICFLFVAAWTPYAVVAMIGAFGDQSL--LTPIASMLPAVFAKTVACFDPYVYAISHPKYRLELSKRVPCL
UVB_megVic RFVPEGFLTSCSFDYLTPT-EETKAFVGTMFVICYVIPMSFIIYFYSQIVCHVFNHEKALREQAKKMNVESLRSNQDAN-AQSAEVRIAKAAITICFLFVAAWTPYAVVAMIGAFGDQSL--LTPIASMLPAVFAKTVACFDPYVYAISHPKYRLELSKRVPCL
UV5_dapPul RYVPEGFLTTCTFDYMTED-ASTRFFVGSIFVYSYVIPLAMLIFYYSKIVRSVGDHEKTLRDQAKKMNVTSLRSNRDQN-EKSAEVRIAKVAIALATLFVFAWTPYAFVALTAAFGNRSV--LTPLLSTVPACCCKLVSCINPWIYAINHPRYRMELQKKMPWF
UV5_braKug RFVPEGFLTTCSFDYITED-SSTRAFVGTIFFTSYVLPMILIIYFYSQIVGHVRQHEETLRAQAKKMNVATLRSGKDDQ-EQSAEVRIAKVCIGLFSMFVISWTPYAAVALLCAFGNRAA--VTPLVSMIPALTCKAVACIDPWIYAINHPRYRLELQKRLPWF
UV5_triLon RFVPEGFLTTCSFDYMTET-SSIRWFVGCIFTYSYIIPLGLIIYYYSKIVGHVQEHERILREQARKMNVESLRSGKDQQ-EKSAEIRIAKVAIGLSLMFVVAWTPYALVALIAAFGNRAV--LTPLVSMIPACCCKAVACIDPWIYAINHPRYRLELQKRMPWF
UV5_triGra RFVPEGFLTTCSFDYMTET-SSIRWFVGCVFTYSYIIPLGLIVYYYSKIVGHVQEHERILREQARKMNVESLRSGRDHQ-EKSAEIRIAKVAIGLSLMFVVAWTPYALVALIAAFGNRAV--LTPLVSMIPACCCKAVACIDPWIYAINHPRYRLELQKRMPWF
UV5_pedHum KFAPEGYLTTCTVDYLTDT-SQTRMFIVTIFFAAYVLPLSLIIYFYTKIVLHVINHEKSLKAQAKKMNVESLRSDGNKN--YAVEIRITKVAIAMCFLFVISWTPYAVVALIGCFGNKHL--ITPLVSMIPACACKAVACIDPYIYAISHPRFRVEVNKRFACL
UVB_anoGam RYIPEGYLTTCSFDYLTDD-PDTRVFVGCIFTWAYVIPMIFICYFYARLFGHVRQHEMMLKNQARKMNVESLTANRSEK-AQAVEMRIAKAAFTIFFLFVCAWTPYAIVTMIGAFGDRTM--LTPFVTMVPAVCCKIVSCLDPWVYAISHPKYRQELERRLPWM
UVB_manSex RFVPEGFLTTCSFDYFTED-QDTEVFVACIFVWSYCIPMALICYFYSQLFGAVRLHERMLQEQAKKMNVKSLASNKEDN-SRSVEIRIAKVAFTIFFLFICAWTPYAFVTMTGAFGDRTL--LTPIATMIPAVCCKVVSCIDPWVYAINHPRYRAELQKRLPWM
UVB_nasVit RYTTEGFLTTCSFDFLSDD-QDTKVFVAAIFSWSYCFPMVLIIYFYSQLIKSVRRHEKMLREQAKKMNVKSL-SAQ-DK-ERSVEMRIAKVAFTIFFLFVCSWTPYAVVTMIAAFGNREL--VTPFSSMLPAVFAKTVSCIDPWVYAINHPRYRQELTKRCQWM
UVB_apiMel RYTTEGFLTTCSFDFLTDD-EDTKVFVTCIFIWAYVIPLIFIILFYSRLLSSIRNHEKMLREQAKKMNVKSLVSNQ-DK-ERSAEVRIAKVAFTIFFLFLLAWTPYATVALIGVYGNREL--LTPVSTMLPAVFAKTVSCIDPWIYAINHPRYRQELQKRCKWM
UVB_diaNig RYVPEGLLTTCSFDYLTDD-EDTKVFTASIFTWSYAFPLCLIVFFYCKLFKQVRLHEKMLQEQARKMNVKSLQTNQDVA-QKSVEIRIAKVAFTIFFLFLCSWTPYATVAMIGAFGNRAL--LTPMSTMIPALFSKIVSCIDPWIYAINHPRFRGELLKRAPWF
UV5B_droMe RYQPEGFLTTCSFDYLTNT-DENRLFVRTIFVWSYVIPMTMILVSYYKLFTHVRVHEKMLAEQAKKMNVKSLSANANAD-NMSVELRIAKAALIIYMLFILAWTPYSVVALIGCFGEQQL--ITPFVSMLPCLACKSVSCLDPWVYATSHPKYRLELERRLPWL
UV5_plePay RYVPEGFLTSCSADFFMQD-FNGRSYIVGTWFFGWFIPVAAIVFFYVQIFLAVKDHEEKIKEQARKMNVDSIRSNEAVK-NSSAEVRIAKTAMCVFLMFLSSWAPYILVAFITGFSDPKLKRITPVISMVPAMTIKASACFDPFFYALSHPRYRLELQNRMPWL
UV5_hasAda RFVPEGFLTSCSSDFYMQD-FNGRSYIVGTWFFGWFIPVAAIIFFYAQIFLAVKDHEEKIKEQARKMNVDSFRSNEALK-NSSAEVRIAKTAMCVVLLFLTSWVPYILVAFIAGFSDPKLKRVTPVISMIPAMTIKGSACFDPFFYALSHPRYRLELQNKLPWL
LWS_anoGam RYVPEGNMTACGTDYLTDD-WFHKSYILVYSVFVYYTPLFTIIYAYFFIIKAVSAHEKNMREQAKRMNVQSLRSSDDGK---STEMKLAKVALVTISLWFMAWTPYTVINYTGVF--KTAS-ITPLATIWGSVFAKANAVYNPIVYGISHPKYRAALLRRFPSL
LWS_rhoPro RYVPEGNMTACGTDYLTKN-WVSRSYILVYSVFVYFLPLFTIIYSYFFILQAVSAHEKQMREQAKKMNVASLRSAEAANT--SAEAKLAKVALMTISLWFMAWTPYLVINYSGIF--ETIS-ISPLFTIWGSLFAKANAVYNPIVYAIRHPKYKQALEKKFPSL
LWS_schGre RYVPEGNMTACGTDYLTKD-WVSRSYILVYSFFVYLLPLGTIIYSYFFILQAVSAHEKQMREQRKKMNVASLRSAEASQT--SAECKLAKVALMTISLWFFGWTPYLIINFTGIF--ETMK-ISPLLTIWGSLFAKANAVFNPIVYGISHPKYRAALEKKFPSL
LWS_lucCru RYVPEGNMTACGTDYLSTG-WFSRSYILFYSWFVYFIPLFAIIYSYWFIVQAVSAHEKAMREQAKKMNVASLRSSEAAQT--SAECKLAKVALMTISLWFLAWTPYLVTNYAGIF--DGSK-ISPLATIWSSLFAKANAVYNPIVYGISHPKYRQALQKKFPSL
LWS_triCas RYVPEGNMTACGTDYLTKD-WVSRSYILVYAVWVYFVPLFTIIYSYWFIVQAVAAHEKSMREQAKKMNVASLRSSEAAQT--SAECKLAKIALMTITLWFFAWTPYLVTNFTGIF--EGAK-ISPLATIWCSLFAKANAVYNPIVYGISHPKYRQALQKKFPSL
LWS_manSex RYVPEGNMTACGTDYLSKS-WVSRSYILIYSVFVYFLPLLLIIYSYFFIVQAVAAHEKAMREQAKKMNVASLRSSEAANT--SAECKLAKVALMTISLWFMAWTPYLVINYTGVF--ESAP-ISPLATIWGSLFAKANAVYNPIVYGISHPKYQAALYAKFPSL
LWS_papXut RYVPEGNMTACGTDYLSKS-WLSRSYILVYSIFVYYTPLLLIIYSYFFIVQAVAAHEKAMREQAKKMNVASLRSSEAANT--SAECKLAKVALMTISLWFMAWTPYLVINYTGVF--ETAP-ISPLATIWGSVFAKANAVYNPIVYGISHPKYRAALYQKFPSL
LWSb_apiMe RYVPEGNMTACGTDYFNRG-LLSASYLVCYGIWVYFVPLFLIIYSYWFIIQAVAAHEKNMREQAKKMNVASLRSSENQNT--SAECKLAKVALMTISLWFMAWTPYLVINFSGIF--NLVK-ISPLFTIWGSLFAKANAVYNPIVYGISHPKYRAALFAKFPSL
LWS_homCoa RYVPEGNMTACGTDYLTPD-WISKSYILVYSLFCYFMPLFLIIYSYWFIVQAVSAHEKAMREQAKKMNVASLRSSDAANT--SAEHKLAKVALMTISLWFCAWTPYLVINYAGIF--QALT-ISPLFTIWGSVFAKANACYNPIVYAISHPKYRAALNKKFPSL
LWSa_nasVi RYVPEGNLTACGTDYFSKD-WVSRSYIVVYSFFVYFLPLFMIIYSYYFIIKAVSAHEKNMREQAKKMNVASLRQGDSQ----SAENKLAKIALMTISLWFMAWTPYLVINWAGIF--DLAR-LTPLFTIWGSVFAKANAVYNPIVYGISHPKYRAALFARFPSL
LWS_acyPis RYVPEANMTACGTDYLTLA-WHSRSYVLVYAIFAYYLPLLVIIYAYYFIVKAVASHEKSMREQAKKMNVSSLRSGDQSNT--SAEFKLAKVALMTISLWFMAWTPYMVINFAGIF--QLMT-IDPLFTIWGSVFAKANAVYNPIVYAISHPKYRLALDKKFPCL
LWSb_nasVi RYVPEGNMTSCGTDYVSDD-WLGKSYILVYSIFVYYTPLFSIILCYWHIVSAVAAHERGMREQAKKMNVASLRSGDQSGE--SAEVKLAKVAVTTISLWFLAWTPYLVTNYMGIF--AKQH-VSPLFTIWASLFAKTNACYNPIVYGISHPKYRAGLKVKCPCL
LWSa_apiMe RYVPEGNMTACGTDYLSQD-WTFKSYILVYSFFVYYTPLFTIIYSYYFIVSAVAAHEKAMKEQAKKMNVTSLRSGDNQNT--SAEAKLAKVALTTISLWFMAWTPYLVINYIGIF--NRSL-ITPLFTIWGSLFAKANAIYNPIVYGISHPKYRAALKEKLPFL
LWS6_droMe RYVPEGNMTACGTDYFAKD-WWNRSYIIVYSLWVYLTPLLTIIFSYWHIMKAVAAHEKAMREQAKKMNVASLRNSEADKSK-AIEIKLAKVALTTISLWFFAWTPYTIINYAGIF--ESMH-LSPLSTICGSVFAKANAVCNPIVYGLSHPKYKQVLREKMPCL
LWS_meoOer RYVPRGDMTACGTDYLTED-EFSRSYLYVYSVWVYIGPLALIIYCYFHIVSAVATHEKQMRDQAKKMGVKSLRTEEAKKT--SAECRLAKVALTTVSLWFMAWTPYLIINWAGMF--YPSV-VSPLFSIWGSVFAKANAVYNPIVYAISHPKYRAALYKKLPCL
LWS1_droMe RYVPEGNLTSCGIDYLERD-WNPRSYLIFYSIFVYYIPLFLICYSYWFIIAAVSAHEKAMREQAKKMNVKSLRSSEDAEK--SAEGKLAKVALVTITLWFMAWTPYLVINCMGLF--KFEG-LTPLNTIWGACFAKSAACYNPIVYGISHPKYRLALKEKCPCC
LWS2_droMe AYVPEGNLTACSIDYMTRM-WNPRSYLITYSLFVYYTPLFLICYSYWFIIAAVAAHEKAMREQAKKMNVKSLRSSEDCDK--SAEGKLAKVALTTISLWFMAWTPYLVICYFGLF--KIDG-LTPLTTIWGATFAKTSAVYNPIVYGISHPKYRIVLKEKCPMC
LWS_limPol RYVPEGNLTSCTVDYLTKD-WSSASYVVIYGLAVYFLPLITMIYCYFFIVHAVAEHEKQLREQAKKMNVASLRANADQQKQ-SAECRLAKVAMMTVGLWFMAWTPYLIISWAGVFS-SGTR-LTPLATIWGSVFAKANSCYNPIVYGISHPRYKAALYQRFPSL
LWS_ixoSca RYVPEGNMTSCTIDYLTKA-LWSASYVVAYAGGVYWTPLFINIYCYSKIVRAVAQHEKQLRLQARKMNVASLRANAEQTKT-SAEARLAKIALMTVGLWFMAWTPYLTIAWAGIFS-DGSK-LTPLATIWGSVFAKANACYNPIVYGISHPKYRAALARRFPSL
LWS2_plePa RYVPEGNMTSCTVDYLSED-LKSSSYVLIYGCAVYFIPLFTLIYNYTFIVRAVSIHEDNLREQAKKMNVTSLRANADQQKQ-SAECRLAKIALMTVGLWFIAWTPYLCIAWSGIFS-SRKH-LTPLATIWGAVFAKAVAVYNPIVYGISHPKYRAALFQKFPSL
LWS2_hasAd RYVPEGNMTSCTVDYLTED-LKSSSYVLIYGCAVYFTPLFTLIYNYTFIVRSVSIHENNLREQAKKMNVSSLRANADQQKQ-SAECRLAKIALMTVGLWFIAWTPYLSIAWSGIFS-SRKH-LTPLATIWGAVFAKAVAVYNPIVYGISHPKYRAALFEKFPSL
LWS1_plePa SYAPEGSMTGCTVDYLHTD-ISTMSYLIVYAIFVYFVPLFIIIYCYTYIVMQVAAHEKSLREQAKKMNIKSLRSNEDNKKA-SAEFRLAKVALMTICLWFMAWTPYLILSLLGIFS-DREW-LTPLTSIWGAVFAKAASAYNPIVYGISHPKYRAALHEKFPCL
LWS1_hasAd RYVPEGSMTSCTIDYIDTA-INPMSYLIAYAIFVYFVPLFIIIYCYAFIVMQVAAHEKSLREQAKKMNIKSLRSNEDNKKA-SAEFRLAKVAFMTICCWFMAWTPYLTLSFLGIFS-DRTW-LTPMTSVWGAIFAKASACYNPIVYGISHPKYRAALHDKFPCL
BCRa_hemSa SYTLEGILDSCSYDYFTRD-MNTITYNICIFIFDFFLPASVIVFSYVFIVKAIFAHEAAMRAQAKKMNVTNLRSN-EAETQ-RAEIRIAKTALVNVSLWFICWTPYAAITIQGLL-GNAEG-ITPLLTTLPALLAKSCSCYNPFVYAISHPKFRLAITQHLPWF
BCRb_hemSa NYILEGILDSCSYDYLTQD-FNTFSYNIFIFVFDYFLPAAIIVFSYVFIVKAIFAHEAAMRAQAKKMNVSTLRSN-EADAQ-RAEIRIAKTALVNVSLWFICWTPYALISLKGVM-GDTSG-ITPLVSTLPALLAKSCSCYNPFVYAISHPKYRLAITQHLPWF
BCR_porPel KYILEGILTSCSYDYLTQD-FNTRSYNIIIFVFDYFLPAAIIIFSYVFIVKAIFAHEAAMRAQAKKMNVTNLRSG-EAESQ-RAEIRIARTALVNVSLWFICWTPYALISLQGVL-GDLSG-INLLVTTLPALLARSCSWYNPFVYAISHPKYRLAITQHLPWF
BCR_triGra RYIPEGILDSCSFDYLTRD-SSTKSFGLCLFFFDYVTPLSIIVFAYFHIVRAIFEHEKILREQAKKMNVTSLRSNADQNAQ-SAEIRIAKVALINISLWVAMWTPYATIVLQGLL-GNQEN-ITPLVSILPALIAKSASIYNPVIYAISHPRYRVALQQKLPWF
BCR2_triLo RYIPEGILDSCSFDYLTRD-SSTKSFGLCLFFFDYITPLSIIVFAYFHIVRAIFEHEKILREQAKKMNVTSLRSNADQNAQ-SAEIRIAKVALINISLWVAMWTPYATIVLQGLL-GNQEN-ITPLVSILPALIAKSASIYNPVIYAISHPRYRIALQQKLPWF
BCRa_dapPu KYIPEGILDSCSFDYLTRD-TMTISFTCCLFAFDYCVPLIIIIFCYYHIVRAIVHHEDALRDQAKKMNVSSLRSNADQKSQ-SAEIRVAKIAMMNITLWVAAWTPYAAICLQGAV-GNQDK-ITPLVTILPALIAKSASIFNPVVYAISHPKYRLALQKALPWF
BCR_limPol RYVPEGILNSCSFDYLTRD-WATVSYIMGCWICEYALPLMVIIYCYIFIVKAVCDHERHLREQAKKMNVASLRSNVDTQKA-SAEMRIAKVALVNVLLWVVSWTPYAAIAMIGIA-GDQML-ITPLRSALPALAGKAASVYNPIVYAISHPKFRLAMQKEIPCC
BCR2_braKu NFSPEGLLSTCSFDYLNDNKFHGYFYTMYIFTGAYCVPMLLLMFFYSQIVKAVWAHEASSRAQAKKMNVESLRSNADANAE-SAEMRIAKVALTNVLLWVCIWTPYAFVAVTGAF-GNRQI-LTPLVAQLPSLICKMASCLNPLVYAISHPKYRQVLQKELPWF
BCR3_braKu DFSPEGMLSTCSFDYLNENRLHGPIFTGYIFFGAYCVPMFLLFFFYSQIVKAVWAHEAALKAQAKKMNVESLRSNADANAE-SAEVRIAKVALTNVLLWICIWTPYAFVAVTGAF-GNRQI-LTPLVAQLPSLICKCASSLNPIVYAISHPKFRQVIQKDYPWF
BCR1_triGr GYALDGMLGTCSFDYVTRT-WNNRSHILAATAFMWVIPVLIIAGCYWFIVQAVFKHEAELKAQAKKMNVASLRSNADQQQV-SAEIRIAKVAITNVVLWLSAWTPFMVISNLGIWADPQQV--TPLVSSLPVLLSKTSCSYNPLVYAISHPKYRECLKTLVPWI
BCR2_triGr NFALDGILNTCSFDYFSRD-MLSMSYIVSACVWAYVIPLIVIIFCYTFIVRAVFEHEETLRQQAAKMNVTSLRSSANSEDT-SAEFRIAKIAMINVCLWLWAWSPFTIVSFIGIF-GNQAI-ITPYLSSLPVILAKTSSVYNPIVYALSHPRYQAALKEEFAWL
BCR1_triLo NFALDGILNTCSFDYFTRD-MPAMSYIVGACVSAYVIPLIVIIVCYTFIVRAVFEHEETLRQQAAKMNVTSLRSSASAEDT-SAEFRIAKIAMINVCLWLWAWSPFTIVSFIGIF-GNQAI-ITPYLSSLPVILAKTSSVYNPIVYALSHPKYQAALKEEFAWL
BCR3_triGr NLSVDGLLNTCSYDYYTRD-LPTVAYIVGSCVHAYVLPLAVIIFCYSYIVQAVFHHERQLREQAAKMNVASLRSSGGKQDEMSAEFRIAKIALINCCLWLWAWTPFTVISFMGVLHDDQSI-INPYVSSLPVLLAKTSAVYNPIVYGLSHPKFQQCLREEFGWN
Consensus r%vpEG.$t.CsfDYlt.. ...r.%....f...y..Pl..!iy.Y..iv.aV..he..lreqakkmnv.slrs..........E.riakva.....Lw..aWtPYav.a..G.f...... .tPl.sm.pa.f.K..ac.#P.vYaisHP.%r.el....p.l
Nematodes ... 3 opsins
Opsins are poorly represented in the nematodes with genetic data. In particular, the complete genome of the model organism C. elegans has no opsins among its GPCR. While it is sometimes argued that a fruit nematode has no need for photoreception, the rat intestinal parasite Strongyloides ratti does in fact have at least two ERY K-296 ciliary-type opsins despite a dark environment for most of its life cycle. However photoreception could be useful during the free-living stage. Ocelli have long been known in marine nematodes.
These genes are very diverged and not informative for bilateran opsin evolution but do have support from an old transcript from Parastrongyloides (BI322533). Their best match is to TMT ciliary opsins in arthropods though those matches are poor (27% identity).
>OPSN1_strRat Strongyloides ratti (nematode) genomic 0 MLINNTLSTNIFYLANEDEIDLLEKSFNQHIVSIILSIWMLLIAIIGFFGNGLIAFVFSRKRSLRKKFLLIIVISILYSFGSLFFVLNSYHIYFYKIMKEP 1 2 ICTIHAFAISTLSVGGIHQLTLLSFERYVGVNHPFVSMKVSLKTKVLFATGAFVWTLLTTTPPLFGWSRYKVLDDSLHYCVFDYLSKEKSSQLYILYLFLNAYIIPLSIIGFSNYKIFLAAK 1 2 DNISKNEFFNNHQYYHSLLIYKKQQRKCSRVIFYCVGSFVISWTPYAFTSIVSNIIFNTRMNKNVVILTAVAAKMFVIWNPIIYGLMDRTFKNECIQIFSKIFK* 0 >OPSN2_strRat Strongyloides ratti (nematode) genomic 0 MYNNTFDEEFFGTSLTSTIKSLEFKDTANGTIFLSFCMIIIVFFGFFGNGLIVYAFIRDGHLRKKNFLLIVISTLYAFGSLFFTLDIYHLYNNKVMEDP 1 2 ICTIHAFAISTLSVGGIHQLSVMSLERYVGVNHPFILMKLPLRTKVLFATGAFVWTLLTTTPPLFGWSRYKVLDDSLHYCVFDYLSKEKSTQKYIMYLFFNGYILPLSIIGFSNYKIFLAAK 1 2 KKICESTGLYDSETQQSLSVYDKQQRKCATVIFFCVGSFIISWTPYAFTSIVWNIIFNYKLNPTFVLLTAIAAKMFVIWNPIIYGFMDKSFQRECEKILFKKFNYWLE* 0 >OPSN2_parTri Parastrongyloides trichosuri (nematode) BI322533 transcript METNVTNSVLNSTLKATSVSVLETTSLPSQFKDTSLGVAVLCFCMILIVIIGFVGNSMIVFIFGRKPHLRKKNFLLIVISVLYASGSLFFSLDIYHLYHHKVMVDPVCTIHAYAISTLCvGGIHQLALLSFERYIGI
See also: Curated Sequences | Lophotrochozoa | Deuterostomes | Cnidaria | Update Blog